Here are today’s class notes. These notes cover intermolecular forces in liquids and how they affect liquid properties. They also cover the kinds of forces present in the solid state.
Category Archives: CHM 111 Class Notes (Spring 2009)
CHM 111: Board notes from 1/21/09
Here are the notes from Wednesday’s CHM 111 class. These notes continue our discussion of valence bond theory from Chapter 10, then introduce the phases of matter from Chapter 11.
We discusses several chlorine-containing hydrocarbons during our talk about how double bonds work in valence bond theory. Here they are.
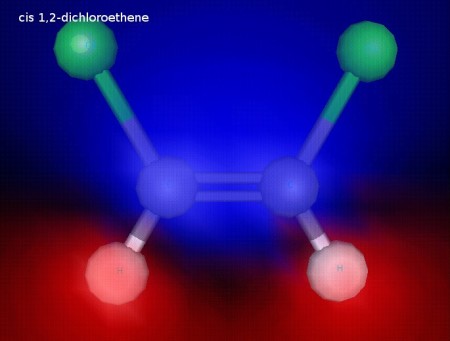
This is cis 1,2-dichloroethene. Both of the chlorine atoms are on the same side of the C=C bond. The chlorine atoms are fixed in this position because the double bond does not allow free rotation, since rotation would break the pi component of the double bond. This illustration shows electrostatic potential, and we can see that this molecule is polar.
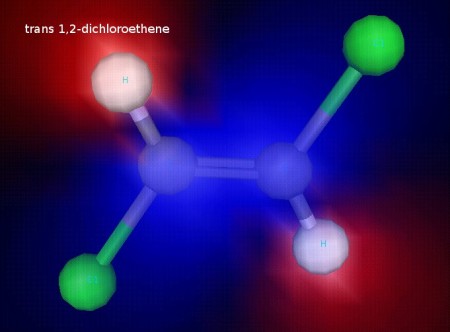
This is trans 1,2-dichloroethene. The chlorine atoms are on opposite sides of the C=C bond. The chlorine atoms are fixed in this position because the double bond does not allow free rotation, since rotation would break the pi component of the double bond. This illustration shows electrostatic potential, and we can see that this molecule is nonpolar. It also has observably different physical properties (like boiling point) from the cis form.
CHM 111: Board notes for 1/14/09
Here are the notes from today’s CHM 111 class. These notes discuss polarity and molecular shape, and introduce valence bond theory.
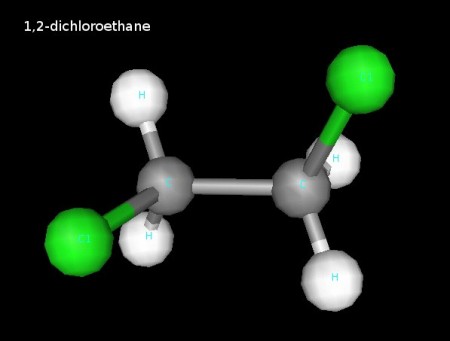
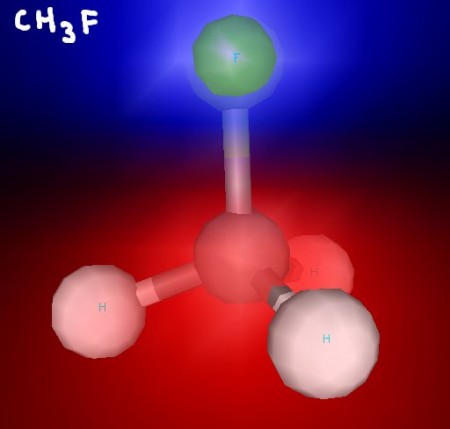
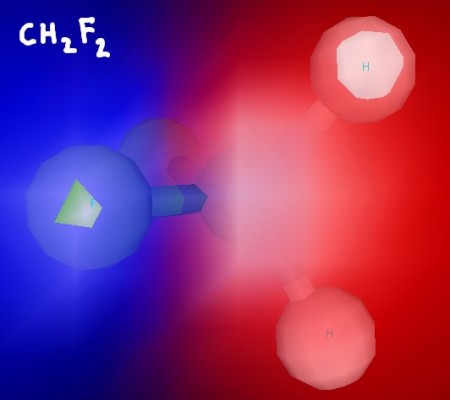
Here area few 3D renderings of some of the molecules we discussed in class. You can click each image for a larger version.
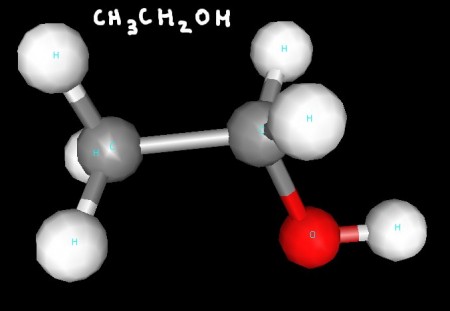
Ethanol's two carbons are both tetrahedral, while the geometry around the oxygen atom is bent (a derivative of tetrahedral).
CHM 111: Board notes for 1/12/09
Here are the notes from today’s CHM 111 class. These notes discuss VSEPR and molecular shape for simple molecules.
Please click the link and make sure that the typed text appears on the notes. If it doesn’t, please leave a comment and describe what you do see when you click the link.
If you’re prompted to install the Adobe Reader plugin, then install it. (That’s normal if you’ve never installed Adobe Reader for anything else. School computers should already have Adobe Reader installed.)