Here are the notes from today’s CHM 111 class. These notes discuss polarity and molecular shape, and introduce valence bond theory.
[CHM 111: 1/14/09 notes]
Here area few 3D renderings of some of the molecules we discussed in class. You can click each image for a larger version.
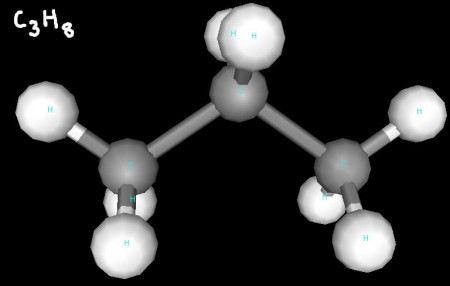
You can see that each of propane's three carbon atoms have a tetrahedral geometry.
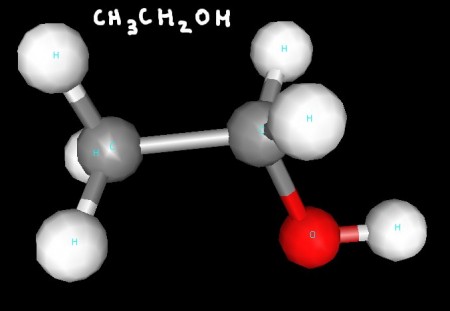
Ethanol's two carbons are both tetrahedral, while the geometry around the oxygen atom is bent (a derivative of tetrahedral).
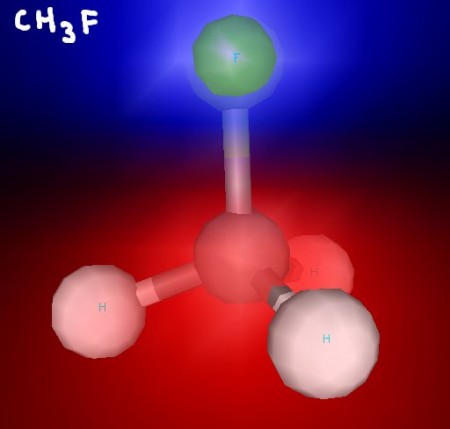
This molecule is polar, since fluorine is able to draw electrons towards itself. This picture is shaded to show electrostatic potential: blue for negative, and red for positive.
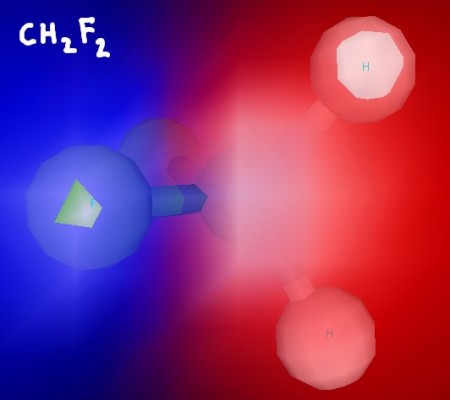
Even thought it's not immediately obvious from the Lewis structure (see the notes for the structure), this molecule is polar. In three dimensions, you can clearly see that the fluorine atoms are on the same side of the molecule.




