Here are the notes from Wednesday’s CHM 111 class. These notes continue our discussion of valence bond theory from Chapter 10, then introduce the phases of matter from Chapter 11.
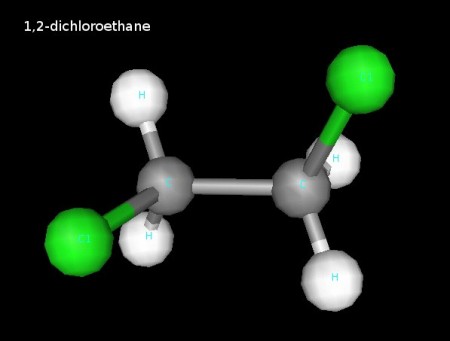
We discusses several chlorine-containing hydrocarbons during our talk about how double bonds work in valence bond theory. Here they are.
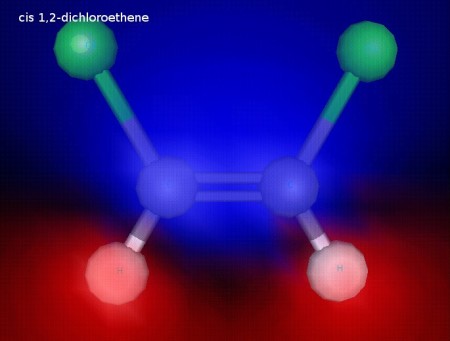
This is cis 1,2-dichloroethene. Both of the chlorine atoms are on the same side of the C=C bond. The chlorine atoms are fixed in this position because the double bond does not allow free rotation, since rotation would break the pi component of the double bond. This illustration shows electrostatic potential, and we can see that this molecule is polar.
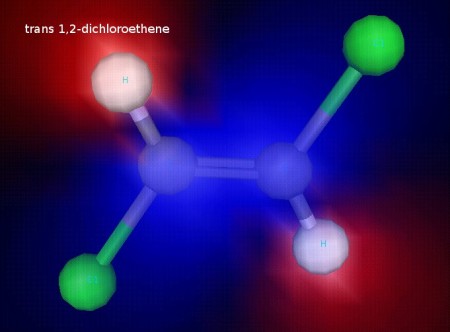
This is trans 1,2-dichloroethene. The chlorine atoms are on opposite sides of the C=C bond. The chlorine atoms are fixed in this position because the double bond does not allow free rotation, since rotation would break the pi component of the double bond. This illustration shows electrostatic potential, and we can see that this molecule is nonpolar. It also has observably different physical properties (like boiling point) from the cis form.