Here are today’s CHM 111 notes, covering polarity of molecules and introducing valence bond theory.
Any questions? Ask here!
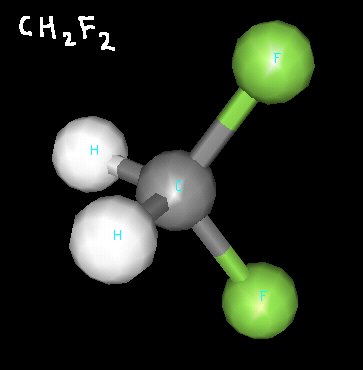
Click the link below for 3D renderings of some of the molecules we’ve discussed in class recently.
Carbon tetrachloride, a tetrahedral molecule
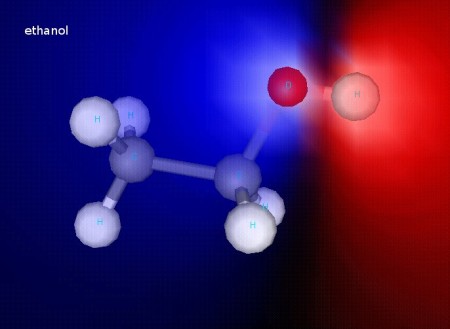
 Ethanol
Ethanol
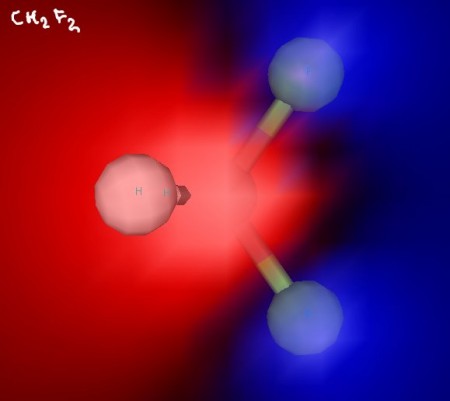
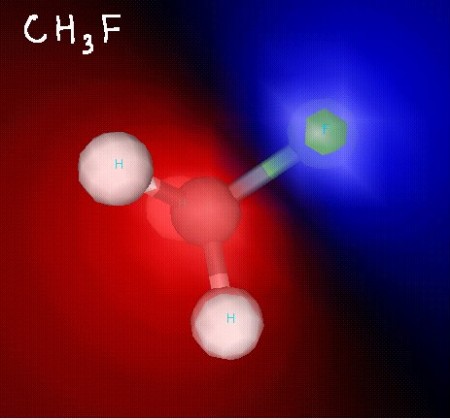
Below, you’ll find some structures for molecules we discussed while talking about polarity.
Ethanol, as you can see in this shaded image, is polar. That helps explain why ethanol and water mix so well together!