Here are a few three-dimensional renderings of some of the molecules we talked about in today’s class. Click the link below to see the renderings.
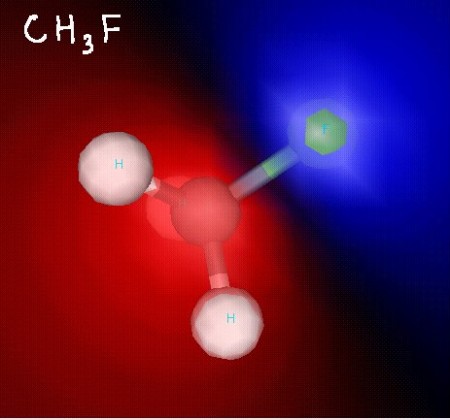
Here’s CH3F. This molecule has been shaded so that areas of lower electron density are red, while areas of higher electron density are blue. You can clearly see that this molecule is polar!
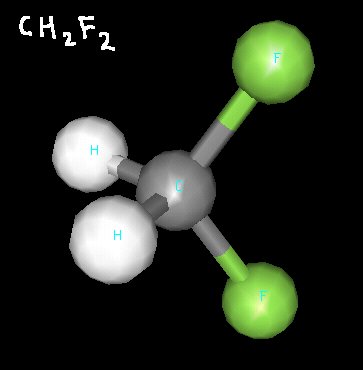
The next molecule is CH2F2. In three dimensions, you can see that this molecule has a hydrogen “side” and a fluorine “side”, making it polar.
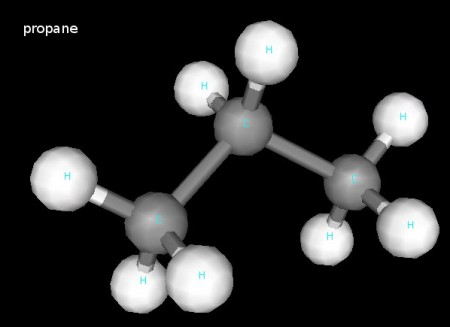
Here’s the propane molecule, C3H8. Observe the three tetrahedral carbon atoms.
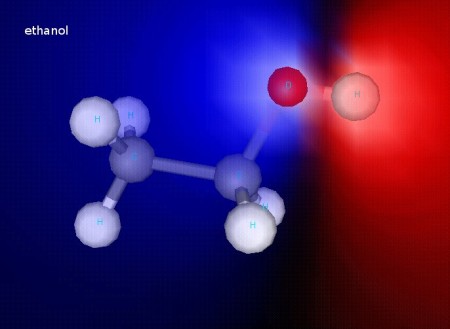
Ethanol, as you can see in this shaded image, is polar. That helps explain why ethanol and water mix so well together!
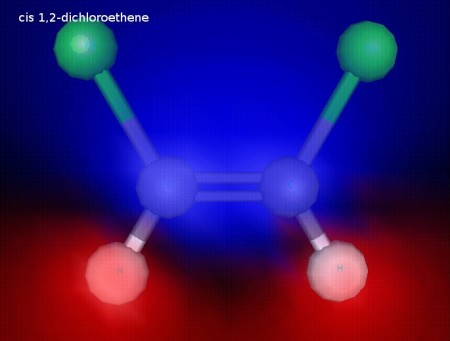
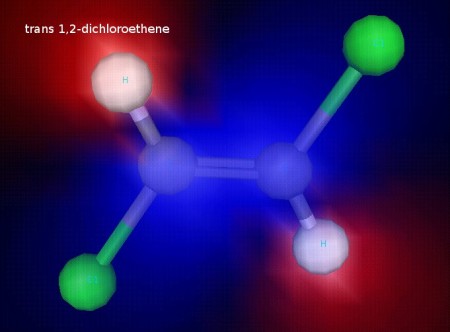
cis 1,2 dichoroethene is polar. trans 1,2-dichloroethene is not. You can see in these images that the cis form has a positive and a negative side, while the trans form does not (Try to find a side of the transform that’s all blue with red on the other side!)
In case you were wondering, these images were all created with free software called Ghemical. Ghemical is written to run under Linux and other unixlike operating systems, but is also available for Windows.