Here are today’s CHM 111 notes, covering polarity, valence bond theory, hybridization, and multiple bonds. At the end, you’ll find a quick overview of the molecular picture of solids, liquids, and gases.
Any questions? Leave a comment here!
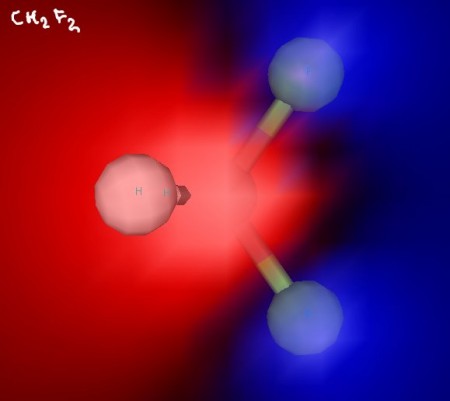
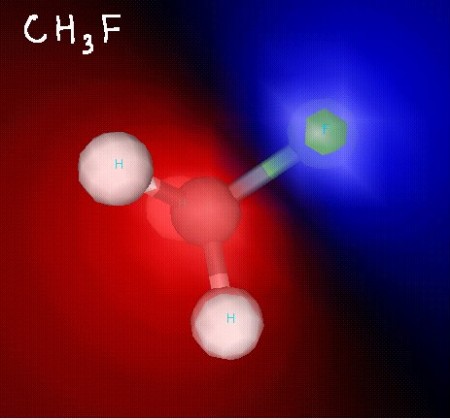
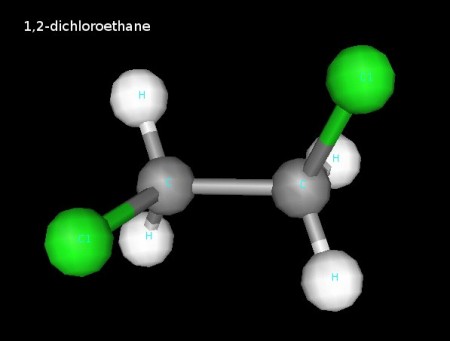
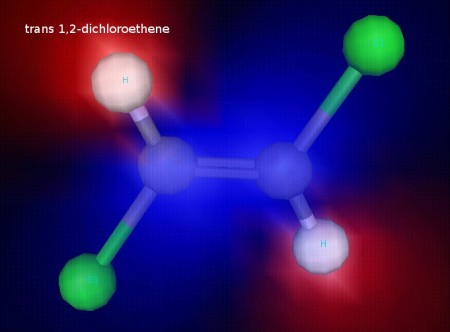
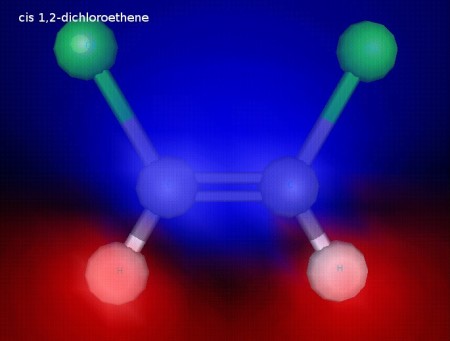
Click the link below to see 3D renderings of some of the molecules we discussed in today’s class.
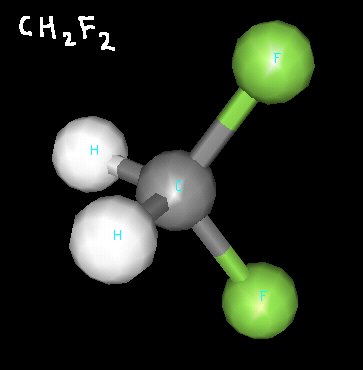
Difluoroethane, showing that the fluorine atoms and hydrogen atoms are on different sides of the molecule