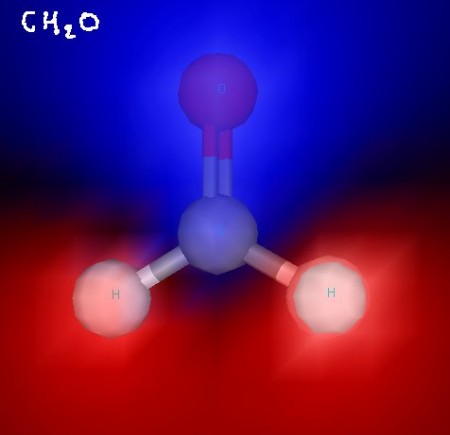
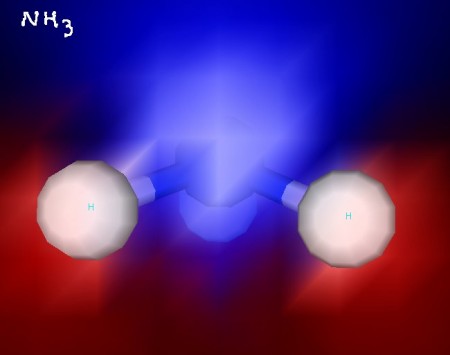
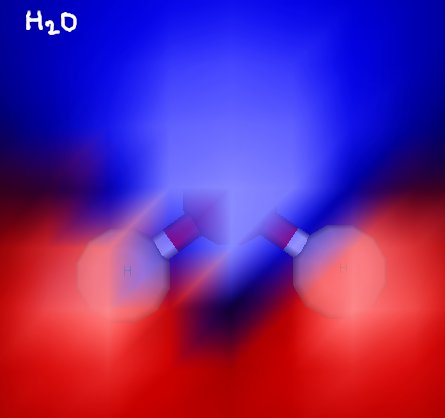
Here’s formaldehyde. You can see the trigonal planar structure clearly. In some of these images, electrostatic potential is indicated with blue (for negative) and red (for positive) to show the polarity of these molecules. Formaldehyde is a polar molecule.
Take a look at the pyramidal shape of ammonia. Its nitrogen atom is the top of the pyramid, while its hydrogen atoms form the base. Ammonia is a polar molecule.
Water is a bent molecule, which gives the molecule a hydrogen “side” and an oxygen “side”. This makes the molecule polar.
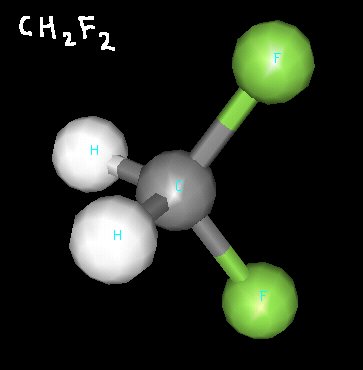
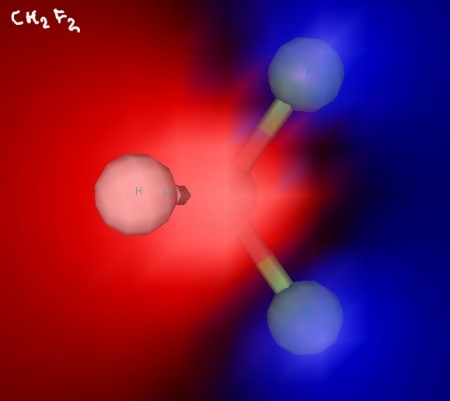
Difluoromethane is a molecule that, at first glance, may appear nonpolar. It has two hydrogen atoms and two fluorine atoms, but their tetrahedral arrangement around the central carbon atom makes the molecule have a hydrogen “side” and and fluorine “side”.
Since fluorine atoms are very electronegative, their arrangement makes difluoromethane a polar molecule.