The dark gray areas represent the 1s orbital of the hydrogen atom.
The black dot represents the nucleus of the hydrogen atom (not to scale).
Each hydrogen has one electron in this orbital.
Illustration 1: Two hydrogen atoms
Introduction
We've discussed Lewis structures and the VSEPR model of bonding. Both of these are models - that is, they just show how atoms tend to bond. What they don't do is provide a theoretical explanation for why the molecules are put together that way. As scientists, we'd like to be able to have a theory that can give an explanation for why atoms bond as they do. We'd also like to use the theory to predict observable properties other than structure!
There are many theories that explain bonding - some simple, some complex. None are perfect. As beginning chemists, we'd prefer a simple theory that we can use to explain most things we'd encounter in the lab. The simplest generally useful theory of bonding is called valence bond theory, and we will briefly discuss it. Valence bond theory is not perfect, and fails to completely describe quite a few molecules (like the O2 molecule), but it is good enough for us at this point.
Valence bond theory: what is it?
What is valence bond theory? From the name, we know that this theory involves the valence electrons of an atom - that is, the electrons in the atom's outermost atomic orbitals. Valence bond theory has this to say about how bonds are formed:
Bonds are formed when two atoms are close enough together so that their orbitals overlap. In other words, electrons from both atoms may be in a region of space which is claimed (or shared, if you prefer) by both atoms. Remember that we defined covalent bonding as electron sharing.
Each set of two overlapping atomic orbitals can contain at most a total of two electrons. Orbitals containing one electron each can overlap and bond. O rbitals that already contain two electrons can only bond with empty orbitals. A bond between a full orbital and an empty one is called a coordinate covalent bond .
What do these two statements mean? From the first, we get the notion that molecular geometry must be dictated by the overlapping orbitals. From the second, we can see why the smaller noble gases never bond. Where would an atom like neon put any electrons? All its orbitals contain two electrons. Verify that by writing out the electron configuration.
A picture of valence bonding
We'll now look at valence bonding graphically. This should help you visualize the points we just made above. We start with the simplest case possible - the hydrogen atom.
Hydrogen has only one electron, and it's in an "s" orbital. (Hydrogen's electron configuration would be written 1s1.) So, hydrogen would bond with another element when its half-full 1s orbital overlapped with another half-full orbital from another atom. For simplicity, let's look at hydrogen bonding with another hydrogen to form the H2 molecule.
|
|
|
|
Illustration 1: Two hydrogen atoms |
|
To bond, these two hydrogen atoms must come together so that their 1s orbitals overlap and form a bond.
|
|
|
|
Illustration 2: The H2 molecule |
|
This picture agrees with the Lewis dot formula for hydrogen. The Lewis formula has one unpaired electron, suggesting that it will form one bond (which it does). That unpaired electron represents the unfilled "s" orbital.
Hybridization
Let's look at a more complex atom like carbon. Carbon is an important element for life, and it has been extensively studied. Carbon's electron configuration is 1s22s22p2. Carbon has four valence electrons, and if you drew a Lewis formula for carbon, it would have four unpaired electrons. This suggests that it is capable of forming four bonds. In the Lewis structures you've drawn so far, you've seen carbon form four bonds.
Valence bond theory predicts that bonds will be made with half-filled orbitals. If we draw an orbital diagram for carbon, we can see that carbon has two half-filled orbitals and one empty orbital. So how can it form four bonds?
|
|
|
|
Illustration 3 - Orbital diagram for the carbon atom |
|
This is where the notion of hybridization comes in. Hybridization is the combination of atomic orbitals to make new ones that describe the bonding in the molecule. Orbitals themselves are mathematical functions - probability distributions of electrons in an atom. These can be combined with other orbitals mathematically. We're not going to go into the math, but we will describe in qualitative terms how these combinations work.
Let's say we're dealing with carbon in a carbon tetrafluoride molecule: CF4. If we were to describe this molecule, we would say that it is a tetrahedral molecule with carbon at the center. Its Lewis structure would look like this:
|
|
|
|
Illustration 4 - Lewis structure of CF4 |
|
How can we make our valence bond theory agree with this? We allow the valence orbitals to combine and form new orbitals which can bond to form the compound. When we combine orbitals:
The number of electrons on the atom does not change.
The overall number of orbitals does not change. (the type does change, though)
For carbon to bond four times, it needs four unfilled orbitals. In addition, the orbitals need to be identical except for the direction they point in - since all four carbon-fluorine bonds in CF4 have been verified by experiment to be identical. If we combine the three 2p and one 2s orbital into a set of four identical orbitals (which we will call "sp3 hybrid orbitals"), we can form four identical bonds with four fluorine atoms. Our new orbital diagram will look like this:
|
|
|
|
Illustration 5 - An orbital diagram showing the sp3 hybrid orbitals of carbon in CF4 |
|
In graphical form, these hybrid orbitals would look like this:
|
|
|
|
Illustration 6 - A graphical representation of the sp3 hybrid orbitals of carbon in CF4 |
|
Different kinds of hybrid orbitals are formed depending on the geometry of the molecule (including lone pairs - lone pairs are just filled orbitals!). Here's a list of the types of hybrid orbital compared to the shape of the molecule.
|
Type of hybrid orbital |
Number of orbitals used |
Shape of molecule |
|---|---|---|
|
sp |
2 |
linear |
|
sp2 |
3 |
trigonal planar or bent with one lone pair |
|
sp3 |
4 |
tetrahedral, (trigonal) pyramidal, or bent with two lone pairs |
|
sp3d |
5 |
trigonal bipyramidal (and its derivatives) |
|
sp3d2 |
6 |
octahedral (and its derivatives) |
Sometimes "d" orbitals form hybrids and bond. Atoms that violate the octet rule generally bond this way. The hybrid orbitals formed include some empty "d" orbitals. Examples of this are PCl 5 and XeOF 4 . Try drawing Lewis structures for these molecules.
Single bonds and double bonds and triple bonds, oh my!
So far, we've only talked about single bonds. We'd like to apply valence bond theory to double and triple bonds as well. According to valence bond theory, bonds are formed by orbital overlap between atoms. Single bonds are formed by overlap on the axis between the atoms by hybrid orbitals. Double and triple bonds are formed by a different kind of overlap .
First, we'll look at a double bond. Valence bond theory explains any bond as an overlap of orbitals on the bonding atoms. So a double bond involves the overlap of two sets of orbitals (two orbitals from each bonding atom). How does this explain the difference in energy? Let's look at the valence bond picture of the bonding in ethylene - C2H4.
Ethylene has this Lewis structure:
|
|
|
|
Illustration 7 - Lewis structure of C2H4 |
|
We would expect that the carbon atoms in this structure would have sp2 hybrid orbitals. Carbon's orbital diagram then looks like this:
|
|
|
|
Illustration 8 - An orbital diagram of carbon in C2H4 showing sp2 hybrid orbitals |
|
We can represent this graphically. The three sp2 hybrid orbitals on the carbon will be arranged in a trigonal planar geometry. The half-filled p orbital will also be present.
|
|
|
|
Illustration 9 - Bonding orbitals in C2H4 |
|
If we look at the picture of the double bond that valence bond theory provides, we can see that there are two different kinds of bonds making up the double bond in ethylene, We define the two types of bond like this:
Sigma ( 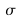 )
bonds: These bonds are formed when orbitals overlap along a
line drawn between the nuclei of the two bonding atoms. You get
this situation when "s" orbitals overlap (as in H2).
You also get this situation when hybrid orbitals overlap, since
hybrid orbitals always point along the lines between the bonded
atoms. Sigma bonds tend to have good overlap between the
bonding orbitals, so sigma bonds are strong .
They're hard to break apart. Single bonds are always
sigma bonds, and double and triple bonds have one sigma bond each .
)
bonds: These bonds are formed when orbitals overlap along a
line drawn between the nuclei of the two bonding atoms. You get
this situation when "s" orbitals overlap (as in H2).
You also get this situation when hybrid orbitals overlap, since
hybrid orbitals always point along the lines between the bonded
atoms. Sigma bonds tend to have good overlap between the
bonding orbitals, so sigma bonds are strong .
They're hard to break apart. Single bonds are always
sigma bonds, and double and triple bonds have one sigma bond each .
Pi
(  )
bonds : These bonds are
formed when orbitals that aren't pointed at each other overlap. Pi
bonds are normally formed when off-axis
"p" orbitals overlap (as in C 2 H 4 ) .
As you can tell from the picture, the overlap in a pi bond isn't as
good as the overlap in a sigma bond, so pi bonds tend to be weaker
than sigma bonds. In other words, it's often easier to break apart
a pi bond than a sigma bond.
)
bonds : These bonds are
formed when orbitals that aren't pointed at each other overlap. Pi
bonds are normally formed when off-axis
"p" orbitals overlap (as in C 2 H 4 ) .
As you can tell from the picture, the overlap in a pi bond isn't as
good as the overlap in a sigma bond, so pi bonds tend to be weaker
than sigma bonds. In other words, it's often easier to break apart
a pi bond than a sigma bond.
What about triple bonds? How does valence bond theory describe the triple bond? The Lewis structure of C2H2 (commonly called acetylene) contains a triple bond.
|
|
|
|
Illustration 10 - Lewis structure of C2H2 showing a triple bond. |
|
The geometry around the carbon atoms is linear. According to valence bond theory, that would mean each carbon has sp hybrid orbitals (see the chart in this note pack). The orbital diagram for a carbon atom in the C2H2 molecule would look like this:
|
|
|
|
Illustration 11 - An orbital diagram showing the sp hybrid orbitals of carbon in C2H2 |
|
Without some 3D plotting software, the picture would look too cluttered on this page – so we'll visualize it in our minds. Think about how the carbons in C2H2 are bonded. How do the sp hybrid orbitals bond? One of the sp hybrid orbitals bonds with the hydrogen. This is a sigma bond. The other sp orbital bonds with an sp orbital on the other carbon - another sigma bond. That leaves the p orbitals. As in the case of the double bond, the p orbitals form pi bonds. This time, we have two pi bonds. These are practically identical except for the direction the p orbitals face. One set points up-and-down and the other set points in-and-out.
For practice, describe the bonding of the carbon atom in carbon dioxide (CO2) using valence bond theory. (Hint: Draw the Lewis structure first, find the shape of the molecule, and then describe the bonding!)
Double bonds and rotation: Support for the valence bond theory
In a compound containing only single bonds, atoms are able to rotate about each other without a chemical reaction. In molecules like ethane (C2H6) and 1,2-dichloroeethane (C2H4Cl2), these rotations require little energy and happen rapidly.
|
|
|
|
Illustration 12 - Two views of C2H6 |
|
|
|
|
|
Illustration 13 - Two views of C2H4Cl2 |
|
Rotation in both of these molecules is about the carbon-carbon single bond in the center.
Now consider 1,2-dichloroethene (C2H2Cl2). There are actually two distinctly different molecules with that name (unlike 1,2-dichoroethane, which represents only a single kind of molecule. They are called isomers, since they have the same chemical formula but a different arrangement of atoms.
|
|
|
|
Illustration 14 - cis and trans 1,2-dichoroethene |
|
The cis form has both chlorine atoms on the same side of the double bond, while the trans form has chlorine atoms on opposite sides of the double bond.
These isomers have different physical and chemical properties (polarity being one important difference). The fact that they can exist at all as separate molecules is easy to explain using the valence bond idea of how a double bond forms. The single bond can rotate without changing the overlap of the orbitals that make up the bond, since that overlap is on the axis between the bonded atoms. This is true for the sigma part of the double bond, too. The pi part, on the other hand, is an off-axis overlap. Rotating around the axis between the carbons in, say, cis 1,2-dichloroethylene would require breaking at least the pi part of the double bond. This requires substantially more energy than rotating around the single bond in the 1,2-dichloroethene. In other words, the rotation around a double bond requires a chemical reaction .
Summary
This note pack contains most of the information you need to know about valence bond theory for this course. We have described the basic postulates of valence bonding, and have shown how we can use the theory to explain the existence of a few simple isomers. We've also linked the type of bonding in a molecule to the shape of the molecule, and we've discussed the concept of hybrid orbitals to explain how certain species (like carbon) can form up to four bonds.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.