"Things" around the central atom
Geometry
Name
2
linear
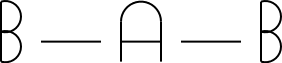
3
trigonal planar
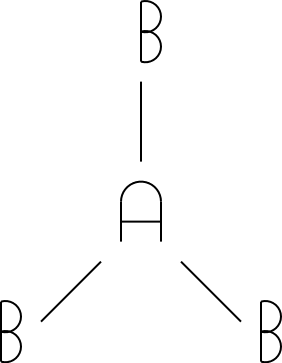
4
tetrahedral
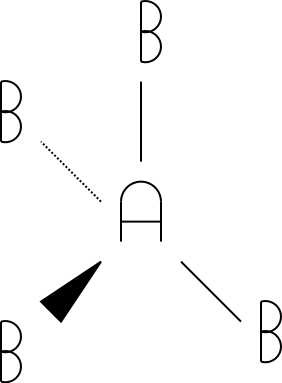
5
trigonal bipyramidal
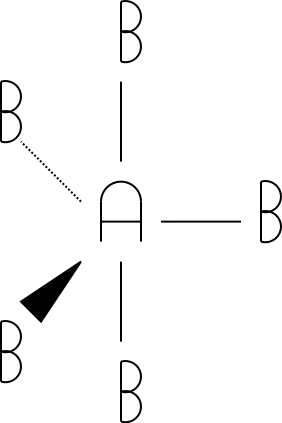
6
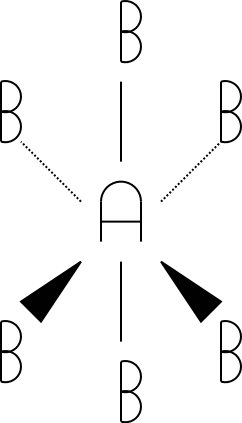
octahedral
Introduction
We have discussed Lewis structures. However, if we are to relate the structure of a molecule to real physical and chemical properties of the molecule, Lewis structures are not enough. We not only need to know which atoms are bonded to each other, but also how these atoms are arranged in space. In short, we need a model that describes how atoms are arranged in molecules.
VSEPR
For most molecules, the Valence Shell Electron Pair Repulsion model (or VSEPR for short) describes how their atoms are arranged. This is a model, so it doesn't try to explain in great detail why the atoms behave the way they do. The model is very easy to understand, since it is based on a very simple concept: like charges repel.
According to VSEPR, electron pairs (in bonded orbitals or as so-called "lone pairs") arrange themselves around the nucleus of an atom so that the pairs are as far apart as possible. You can illustrate this by tying blown-up balloons together - each balloon represents electrons around an atom, and the knot in the center represents the nucleus.
There are five possible overall geometries, shown in the large table below:
|
"Things" around the central atom |
Geometry |
Name |
|---|---|---|
|
2 |
|
linear |
|
3 |
|
trigonal planar |
|
4 |
|
tetrahedral |
|
5 |
|
trigonal bipyramidal |
|
6 |
|
octahedral |
The tetrahedral, trigonal bipyramidal, and octahedral drawings were drawn using a standard convention for drawing molecules in three dimensions. The triangles indicate atoms pointed out of the paper/screen (towards you). The dotted lines indicate atoms pointed into the screen/paper (away from you). Get used to this notation, as you'll see it in future chemistry courses!
Real molecules - with examples
For real molecules, we are interested in how actual atoms are arranged around a central atom. Lone pairs take up space,. They distort the shapes we discussed above. So, to find the true shape of a molecule, we first draw the Lewis structure. We then find the type of geometry around the nucleus of the central atom, remembering that lone pairs do take up space. Finally, we classify the molecule based on the number of other atoms around the nucleus. The more complicated structures (tetrahedral, trigonal bippyramidal, and octahedral) often have some lone pairs distorting them. The table below some variations of the tetrahedral shape.
|
Number of things around nucleus |
Number of atoms around nucleus |
Geometry |
Name |
|---|---|---|---|
|
4 |
2 |
|
bent |
|
4 |
3 |
|
pyramidal |
|
4 |
4 |
|
tetrahedral |
The bent shape above is actually a flat molecule, since the three atoms form a plane. The water molecule has this shape.
After we determine a Lewis structure, we can get the molecule's shape by asking ourselves
What kind of overall geometry - including lone pairs - does the central atom have?
Given that lone pairs take up space, but are otherwise invisible, what is the shape of the molecule? This will be some variation of the geometry you specified for the first question.
Let's do some examples:
Formaldehyde has the chemical formula H2CO. What's the shape of the formaldehyde molecule?
If we draw the molecule, we come up with this structure:
|
|
|
|
Illustration 1: Formaldehyde's Lewis structure |
|
|
|
|
|
Illustration 2: Formaldehyde's 3D structure |
|
There are three things around carbon - two hydrogen atoms and one oxygen atom There are no lone pairs around the carbon atom. The shape of this molecule, then, is trigonal planar .
What's the shape of the ammonia molecule, NH 3 ?
If we draw the ammonia molecule, we come up with:
|
|
|
|
Illustration 3: Ammonia |
|
|
|
|
|
Illustration 4: Ammonia's 3D structure |
|
There are four things around the central nitrogen atom here - three hydrogen atoms and one lone pair of electrons. Since there are four things around nitrogen, we know that the shape of the molecule will be a derivative of the tetrahedral geometry. Three of the four things around the nitrogen are other atoms, so ammonia is pyramidal . (You can see how this is a pyramid by tying three red balloons and one blue balloon together. The three red balloons - representing the hydrogen atoms - will be arranged in a pyramid shape. The lone pair is represented by the blue balloon.)
What is the shape of a water (H2O) molecule?
If we draw the water molecule, we get:
|
|
|
|
Illustration 5: Water's Lewis structure |
|
|
|
|
|
Illustration 6: 3D structure of water |
|
There are four things around the central oxygen atom here - two hydrogen atoms and two lone pairs of electrons. Since there are four things around oxygen, we know that the shape of the molecule will (like the ammonia molecule) be a derivative of a tetrahedral geometry. Two of the four things around the oxygen atom are other atoms, so water is bent.
Large molecules
VSEPR also works for large molecules. Think of a large molecule as a structure made out of smaller parts joined together. Each of the smaller parts has a shape of its own. For example, look at the propane molecule, C 3 H 8 .
|
|
|
Illustration 7: Lewis structure of the C3H8 molecule |
|
|
|
Illustration 8: 3D illustration of C3H8. |
Using VSEPR, you can figure out that the geometry of each of the carbon centers is tetrahedral . The propane molecule is made of three tetrahedral units hooked together. The geometry of particular sites in large biomolecules is important because drugs can be designed to fit into the sites of the biomolecules and react.
Summary
We have discussed the VSEPR model, which gives us a way to figure out how atoms are arranged in molecules. You should now be familiar with the shape of simple molecules (note that more complex molecules are chains of these simple shapes) and how to determine the shape from the Lewis structure. In the future, we will discuss how to relate the polarity of a molecule to its shape. This will let us estimate observable properties of molecules without actually having a sample of the molecule on hand.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.