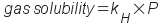Solubility
Introduction
We've discussed previously how solids
and liquids are put together (and how we can get information on the
properties of a liquid or solid starting from as little information
as a chemical formula). Now, we will turn our attention to how
solutions are put together. What makes one substance dissolve in
another one?
A definition of solubility
We already know a little bit about
solubility. Way back near the beginning of the previous course (see
the note pack on electrolytes and the ionic theory of solutions from
chapter 4 for more detail), we talked about the solubility rules,
which we used to guess whether or not a precipitate would form when
we mixed some salt solutions together.
We said something was soluble if
it would dissolve in water, and something was insoluble if it
would not. That's a rather simplistic view that doesn't take into
account other solvents or the fact that sometimes only a certain
amount of one substance will dissolve in another. This is where the
concept of solubility comes in.
Solubility is quantitative - it
is the amount of one substance that dissolves in another and
is usually expressed in units like grams per milliliter (g/mL) or
grams per liter (g/L). We say a substance is "soluble" if
the solubility is large (example - sodium chloride in water). On the
other hand, a substance is "slightly soluble" or
"insoluble" if the solubility is very small (example - a
substance like silver chloride in water).
How and why things dissolve
For this section, we will primarily
look at more familiar solutions like solids in liquids and liquids in
liquids, though our discussion here will be valid for everything
except gas/gas solutions. (There's no real solution process in
a gas, since gas molecules act as if no other molecules are there!).
We start off by asking ourselves this:
"What happens when a substance dissolves?" Consider a
simple example like sucrose (table sugar, C12H22O11)
dissolving in water. A chemical equation for the process might look
like this:
C12H22O11(s)
<--> C12H22O11(aq)
(The double-headed arrow, "<-->",
indicates that this reaction can proceed either way - to the left or
right)
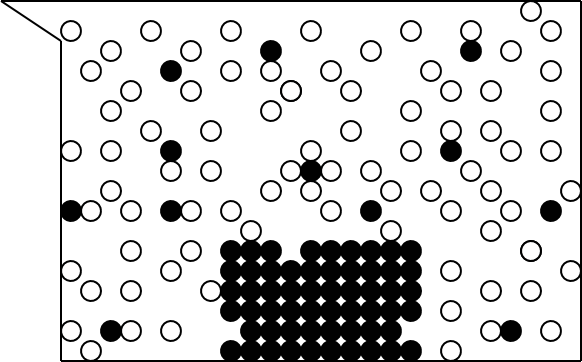
Graphically, we might picture the
solution process this way:
|
|
The sugar molecules are represented by black spheres,
while water molecules are represented by white spheres.
During the solution process, the water molecules pull the
sugar out of the sugar crystals and into solution.
The sugar and water molecules interact in some way.
|
|
Illustration 1 - Sugar in water
|
|
From the picture (which follows from
our pictures of the pure solid and pure liquid), we can see that the
liquid and solid interact. Since what holds a liquid together in the
first place are intermolecular forces, we would reasonably expect
that intermolecular forces also play a role in the solution
process. They do. We can see this role in several sample
situations:
Oil and water don't mix: Oil is
nonpolar, so it's held together by strictly London forces. Water is
different because it is polar and hydrogen bonds.
Sugar and water do mix:
Sugar is a polar molecule, as it water.
Ethanol and water mix extremely
well. Ethanol (CH3CH2OH - see if you can draw
a Lewis structure) and water are both polar and both hydrogen bond.
Sodium chloride and water mix:
Sodium chloride is ionic, while water is polar.
From these four examples and many
others, we can see that substances with similar intermolecular
forces can dissolve each other. In short, "like
dissolves like". Polar substances tend to dissolve in other
polar substances. Nonpolar substances dissolve in other nonpolar
substances. Ionic substances (held together by interactions of full
positive and negative charges) may dissolve in polar substances.
Saturation and supersaturation
For some sets of substances, you can
dissolve any amount of one substance in the other. The substances
are miscible. Gases are completely miscible in each
other - it doesn't matter how much of each gas is present - you can
make a solution from all of it. Certain mixtures of liquids are also
miscible.
For most sets of substances, though,
you reach a point where you can't dissolve any more solute in the
solvent. This point is called the saturation point, and the
solution is said to be saturated.
Another way to define the saturation
point is to say that the substances in solution are in equilibrium.
For a system of sucrose in water, the solution is saturated when the
rate the solid sucrose dissolves is equal to the rate that the
aqueous sucrose precipitates. We know that these processes
occur all the time (like phase transitions) because we can observe
crystals become larger in a saturated solution over time - the
crystals get larger when the solute precipitates on the outside of
crystals already there. Small crystals get smaller and disappear,
and large crystals get larger. We will discuss equilibrium in detail
in later chapter.
Before saturation, we refer to a
solution as unsaturated, meaning that we could dissolve more
solute in the solvent if we wished.
It is possible (under certain
conditions) to form a solution which has more solute than
would be able to dissolve normally in a solvent. These solutions are
called supersaturated, because solute is present at a
concentration greater than the concentration at the saturation point.
These solutions are unstable, though, and even tapping a
beaker containing a supersaturated solution can cause the "extra"
solute to crash out of solution immediately.
Outside factors that affect
solubility
We know that intermolecular forces
effect solubility - we saw that earlier in this note pack. But some
external factors can also affect solubility, and we will discuss two
of these:
1) Temperature: Temperature has
a varied effect on solubility. Increased temperature will either
increase solubility, decrease it, or not affect it significantly.
(How's that for covering all bases?) Luckily, though, there are a
few rules of thumb you can use:
Most (but not all) ionic solids
increase in solubility in water when temperature is
increased. A few decrease in solubility or show no significant
change.
Gases tend to be less
soluble at higher
temperatures. Consider what warm Coke tastes like. It tastes flat
because CO 2
is less soluble in water at room temperature than at the temperature
of your fridge.
A related note: Is the solution
process endothermic or exothermic? Both! For some solutions (like
ammonium chloride in water), the solution process requires energy and
is endothermic. For others (like sulfuric acid in water), the
solution process releases heat and is exothermic.
2) Pressure: Pressure primarily
affects the solubility of gases in liquids. When you increase the
pressure, the solubility of the gas increases. In fact, the
solubility of a gas is directly proportional to its partial
pressure. This is called Henry's Law:
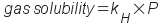
... where kH is the
Henry's Law constant for the gas/liquid solution and P is the partial
pressure of the solute gas.
Summary
In this note pack we have discussed the
solution process. You should understand that solubility is not an
on/off thing - we quantify it be talking about how much solute will
dissolve in a solvent. You should also understand some of the
factors (both the types of forces and the external ones like
temperature and pressure) that influence solubility. You should also
be familiar with basic terms like solubility, saturation, and
supersaturation.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.