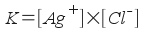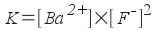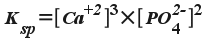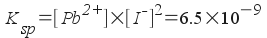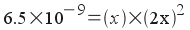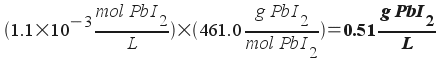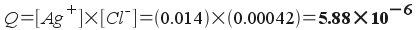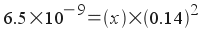Solubility Equilibrium
Introduction
Previously, we learned how to predict
solubility - that is, how to predict (qualitatively, at least)
whether one substance will dissolve in another. Armed with our
knowledge of equilibrium, we'll look at solubility again. This time,
we will discuss the solubility of so-called "insoluble"
salts as an equilibrium process, and we'll use our understanding of
equilibrium to quantify exactly how much of these "insoluble"
salts will dissolve.
Terminology review
Let's review some key terms related to
solubility. A solution is a homogeneous mixture of
substances. Homogeneous mixtures have the same concentration of each
component throughout. A solvent is the component of a
solution present in the greatest amount, and solutes are
components present in lesser amounts that the solute.
A saturated solution contains
the maximum amount of solute that it is normally possible to
dissolve into the solution. You can tell that a solution is
saturated (experimentally) by noting that there will be an amount of
solid at the bottom (this solid wasn't able to dissolve in the
solution) - even if the solution is stirred or left to stand for a
long period.
Saturation and equilibrium
Saturated solutions are, from an
equilibrium standpoint, solutions where solid "solute"
is at equilibrium with solute in solution. For example,
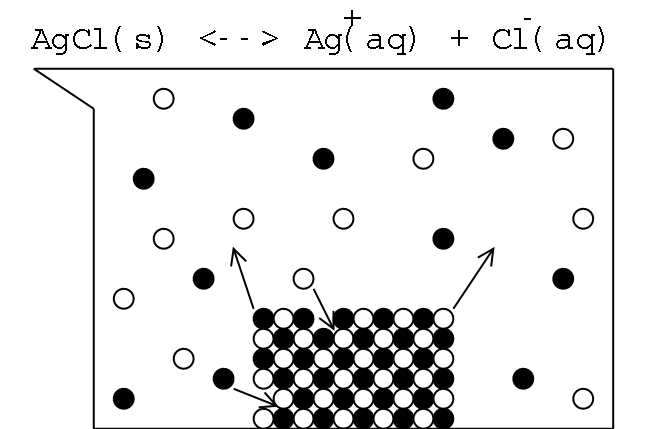
consider a saturated solution of silver chloride (an "insoluble"
salt), AgCl, in water:
|
|
Silver ions are represented by black circles, while
chloride ions are represented by white circles.
Note that ions are continually dissolving out of and
precipitating onto the solid. These processes work at the same
rate in a saturated solution.
|
|
Illustration 1 - Cartoon drawing of saturated AgCl solution
|
|
The equilibrium process pictured above
can be represented with a simple equation:
AgCl(s) <--> Ag+(aq)
+ Cl-(aq)
You would write the equilibrium
constant expression like this:
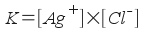
[Note that we leave off AgCl solid
because the concentration of this pure solid
doesn't change even if the amount of pure solid does.]
Similarly, you could look at barium
fluoride, BaF2:
BaF2(s) <-->
Ba2+(aq) + 2F-(aq)
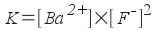
The form of these equilibrium constant
expressions is interesting. It's merely the product of the
concentrations of the ions in solution raised to the power of the
number of that ion present in the salt. For this reason, these
expressions are called solubility product constant expressions
and the constant itself is called the solubility product constant,
Ksp.
Let's try another. What is the
solubility product constant expression for calcium phosphate?
The
chemical formula of calcium phosphate is Ca 3 (PO 4 ) 2 ,
and when it dissolves in water:
Ca3(PO4)2(s)
<--> 3Ca2+(aq) + 2PO43-(aq)
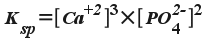
So the solubility product constant is
really nothing new. It's just an equilibrium constant that is
useful enough to be given a special name. Since it's an equilibrium
constant, we can apply all of our knowledge of how equilibrium works
to the solubility of salts in water.
A simple calculation: The solubility
of a salt in water
Let's say we want to know the
solubility of a salt in water. The solubility is, of course,
the amount of the salt that dissolves in a given amount of water
at equilibrium. In other words, it's the amount of salt that is
dissolved in a saturated solution. If we know the solubility product
constant (which is tabulated for many salts in various books, and in
Appendix G of your textbook), we can easily calculate the solubility
of the salt. Let's do an example.
What is the solubility of lead
iodide, PbI2, in 25 ° C
water? Express your answer in terms of grams per liter. Ksp
= 6.5x10-9
To solve this
problem, treat it as a standard equilibrium problem. Write out the
equilibrium reaction and the expression for Ksp:
PbI 2 (s)
<--> Pb 2+ (aq)
+ 2I-(aq)
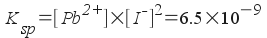
Now, we define all concentrations in
terms of one variable:
Now plug in to the equilibrium
expression and solve:
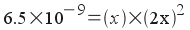
(Note: the 2 in front of the x above
is inside the parenthesis. A common mistake in
solving one of these problems is to forget to square the 2 when
solving.)
6.5x10-9 = 4x3
x = 1.1x10-3
M = [Pb 2+ ]
So
how does this number relate to the solubility? For every mole of
PbI 2
that dissociates , one mole of Pb2+ ions is
produced. So, this number is equivalent to the molar solubility
(solubility expressed as a molar concentration). The problem,
though, asks us for the solubility expressed in grams per liter. To
find the requested answer, simply use the formula weight to convert
from grams to moles!
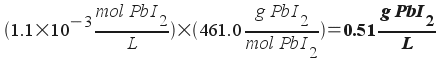
So, the solubility of the PbI2
is 0.51 g/L.
Precipitation and the solubility
product constant
An
application of the solubility equilibrium is gravimetric
analysis .
Gravimetric analysis is a method that
lets you determine how much of a given substance is present in a
sample by reacting it with a precipitating agent. A
precipitating agent is simply a compound that causes another
to precipitate.
For example, a common method for
determining the amount of chloride ion in a sample involves
dissolving the sample in water and adding silver nitrate (AgNO3,
a source of the silver ion Ag+) to precipitate the
chloride as the silver salt. Silver chloride is an "insoluble'
salt:
AgCl(s) <--> Ag+(aq)
+ Cl-(aq) ; Ksp = 1.8x10-10
To precipitate silver chloride, you
must first create a saturated solution. Silver chloride (or any
other salt, for that matter), will not precipitate from solution
unless that solution is saturated. This is one reason why, in a
gravimetric analysis, the procedure will often tell you to add a
large amount of the
precipitating agent.
How do you know when a precipitate
will form, given concentrations of ions in solution? If you
realize that precipitation can occur only after the solution has
enough dissolved ions to be at equilibrium, then it's easy.
In a previous chapter, you calculated a
reaction quotient, Q, to determine whether or not a reaction
was at equilibrium. You can use the reaction quotient Q (sometimes
called the ion product) to determine whether or not a salt
will precipitate from solution. Let's do a quick example.
Will silver chloride precipitate
from a solution that is 0.014 M Ag+ and 0.00042 M
Cl-?
To answer this question, simply find
the reaction quotient. Remember that the reaction quotient has the
same form as the equilibrium constant, so:
AgCl(s) <--> Ag+(aq)
+ Cl-(aq) ; Ksp = 1.8x10-10
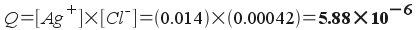
What do you do with Q? Compare it with
Ksp:
If Q < Ksp ,
then the reaction will proceed to produce more products - i.e.
dissolved ions. Therefore, no precipitation will occur.
If Q = Ksp ,
then the reaction is at equilibrium - i.e. you have a saturated
solution. Precipitation is just beginning.
If Q > Ksp,
then the reaction will proceed to produce more reactants - i.e.
precipitate. Therefore, precipitation will occur.
In our example, Q > Ksp,
so precipitation of AgCl will occur.
The common-ion effect
We discussed the common-ion effect when
we discussed acids and bases. We noted that the presence of an ion
that was a product of the dissociation of a weak base or acid
suppressed the dissociation of that weak base or acid. Let's use the
same logic and apply it to the solution process.
What would be the molar solubility
of lead iodide in a solution of 0.14 M sodium iodide?
The solubility equilibrium and Ksp
expression are the same as for lead iodide in pure water:
PbI 2 (s)
<--> Pb 2+ (aq)
+ 2I-(aq)
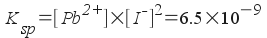
How does the sodium iodide (NaI) figure
in? It's a source of the iodide ion ,
a product in the equilibrium. We'd expect (because of Le Chateleir's
principle that if we added sodium iodide to a saturated solution of
lead iodide, the equilibrium would shift to the left to produce more
PbI 2 .
Logically, then, we should expect that less lead iodide
would dissolve in a solution of sodium iodide than in pure water .
Let's verify this logic by calculation.
Like any
equilibrium problem, we express all concentrations in terms of one
variable.
[Pb2+]
= x
[I - ]
= 0.14 + 2 x
Since the amount of lead iodide that
dissociates is small (Ksp = 6.5x10-9) - and
likely to be even smaller due to the presence of iodide already in
solution - we will assume that 2x is small compared to 0.14.
Now, we plug in and solve just as we
did in the case of pure water:
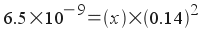
x = 3.3x10-7
M = [Pb 2+ ]
= the molar solubility of PbI2 in a 0.14
M NaI solution.
Comparing the molar solubility of PbI2
in NaI (3.3x10 -7
M )
to PbI2 in pure water (1.1x10-3 M ) ,
we see that the lead iodide is more than 3000 times more soluble
in water than it is in 0.14 M NaI. This is, of course, a result
of the common-ion effect.
Le Chateleir's principle: How pH and
complex formation affect solubility
In addition to the common-ion effect,
Le Chaleleir's principle can tell us whether or not other chemicals
will change the solubility of a substance. Let's consider pH first.
As we already know, some salts have
acidic or basic properties. They have these properties because the
ions that make up the salt can be acidic, basic, or neutral. The
solubility of acidic or basic salts depends on the pH of the solution
you're trying to dissolve them in! As an example, let's look at
magnesium hydroxide, a fairly insoluble salt featured in one of the
CHM 111 labs.
Mg(OH)2(s) <-->
Mg2+(aq) + 2OH-(aq)
The solubility of this salt is very
dependent on pH. Why can we say this? Well, the salt forms two ions
when it dissolves - magnesium ion (a pH-neutral ion) and hydroxide
ion (a basic ion - it can accept a proton to form water). What
does Le Chateleir's principle say about the solubility of this salt
in solutions of different pH values?
In a basic solution, the
concentration of hydroxide is high. Since OH- is a
product in the solubility equilibrium, the equilibrium is forced to
the left. Less Mg(OH)2 dissolves in a
basic solution than in pure water .
In an acidic solution, the
concentration of hydroxide is low. In fact, hydroxide ions produced
by the dissolution of magnesium hydroxide may be converted to water
by the hydronium ions present in the acidic solution. Since OH-
is a product in the solubility equilibrium, the equilibrium is
forced to the right. More Mg(OH)2
dissolves in an acidic solution than in pure water .
Generalizing to all salts,
Basic salts are more soluble in
acidic solutions and less soluble in basic solutions.
Acidic salts are more soluble
in basic solutions and less soluble in acidic solutions.
The solubility of a neutral
salt is not affected by pH.
The formation of complex ions can
also affect the solubility of certain salts. How? Consider the
insoluble salt silver bromide, AgBr.
AgBr(s) <--> Ag+(aq)
+ Br-(aq)
It's known that the silver ion can form
a complex with ammonia via a Lewis acid/base reaction:
Ag+(aq) +
2NH3(aq) <--> Ag(NH3)2+(aq)
So what's the effect of the presence of
ammonia on the solubility of silver bromide? The solubility goes
up, because the ammonia reacts with silver ion and lowers its
concentration. Le Chateleir's principle says that the silver bromide
solubility equilibrium will shift to the right because the
concentration of silver ion has been lowered - so more silver bromide
will dissolve.
Silver salts are so much more
soluble in the presence of ammonia that a way to remove insoluble
silver salts (AgCl, AgBr, etc.) from chemical glassware is to rinse
the glassware with concentrated ammonia!
Generalizing, if an ion in a salt
forms a complex in solution, the solubility of the salt will be
increased.
Summary
In this note pack, we discussed
solubility as an equilibrium process. We defined terms related to
solubility and learned the names used to describe the equilibrium
constant and reaction quotient when dealing with salt solutions. We
discussed that saturated solutions were solutions at equilibrium, and
we discussed the conditions necessary for the precipitation of salts
from a solution. We also discussed Le Chateleir's principle and its
applications to solubilty: the common ion effect, pH, and complex
formation. You should understand how to do simple solubility
calculations (including calculations involving the common-ion
effect). You should be able to predict at what pH conditions a given
salt is most (or least) soluble. You should also be able to
determine whether a precipitate will form given concentration of the
ions in a solution.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.