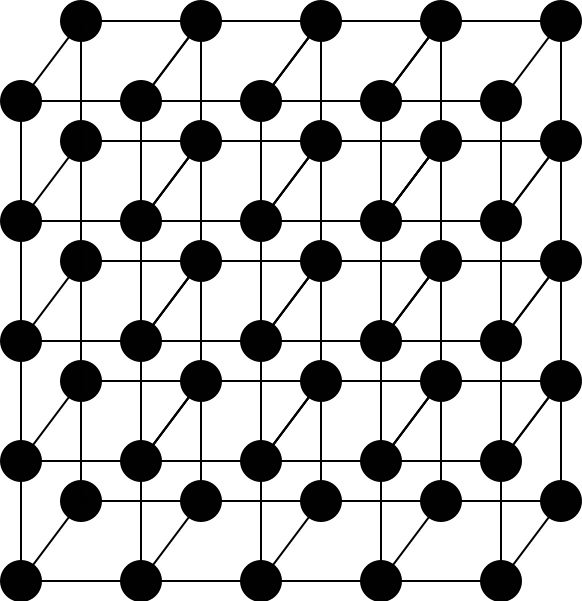
This crystal lattice has lattice points at the corners of a cube.
The entire crystal structure is made of this pattern repeated over and over again.
The unit cell of this lattice is one of the cubes.
Illustration 1: A simple cubic crystal lattice
Introduction
Now that we have discussed liquids in detail, we will turn our attention to solids. Solids as a class are more diverse (in observable properties) than liquids. We will classify solids both by the types of forces that hold them together as well as by structure. We will relate these classifications to several physical properties of solids.
The properties of solids
We've learned several properties of the solid phase:
Solids are rigid - they don't change shape unless you apply a substantial force to them (and sometimes not even then).
Solids are dense - the molecules in a solid are packed together more closely than they are in a gas, and usually more close than they are in a liquid.
Solids are incompressible - you can increase the pressure on a solid without change in the volume of a solid.
There are some properties that are unique to solids. One is the melting point , which is the temperature that the solid changes to a liquid .
Another property is the hardness/brittleness of a solid. It is difficult to move the atoms of a hard material relative to one another. A brittle material fractures when hit hard enough.
A third property is the conductivity - that is, does the material conduct an electrical current?
Classification of solids - Forces
Solids are, like liquids, held together by attractive forces. We can have an idea of the properties of the solid if we know what kind of forces hold the solid together. Here are the classifications:
1) Molecular solids : The atoms or molecules in molecular solids are held together by the same kinds of forces that hold materials in the liquid phase together - van der Waals forces (dipole-dipole interactions, London forces) and hydrogen bonds. Examples of this class of materials are ice and candle wax.
2) Metallic solids: These solids are held together by metallic bonds. A unique feature of metallic solids is their ability to conduct electricity since electrons are free to move throughout the metal solid. Examples of this class of solid are iron, lead, and magnesium metals. (Basically, the solid phase of any metal or alloy.)
3) Ionic solids : Ionic solids are held together by ionic bonds . In other words, they are held together by the interaction of positively and negatively charged ions. Examples of these solids are sodium chloride, potassium nitrate, and ammonium nitrate. The solid phase of any ionic compound is an ionic solid.
4) Covalent network solids : These solids are held together by covalent bonds between the atoms in the solid. Effectively, covalent network solids are gigantic molecules. An example of a common covalent network solid is a diamond.
Classification of solids - Structure
Solids may also be classified by their structure. The classification of solids by structure is a large area of study, and entire classes are offered on ways to do this. Since we're only interested in the big picture at the moment, we will divide solids into two large categories:
1) Amorphous solids: Amorphous solids have a disordered structure without a well-defined arrangement of atoms/molecules. Very few solids are truly amorphous, but quite a few solids do not have an extremely well-ordered structure. A good example of these are certain plastics.
2) Crystalline solids : These solids have a well-defined three-dimensional structure. These solids are composed of one or more crystals .
More on crystals
The structure of a crystal is defined in terms of a crystal lattice , which is a three-dimensional arrangement of points in space. The points are called lattice points , and you can describe a crystal in terms of the arrangement of these lattice points.
|
|
|
|
Illustration 1: A simple cubic crystal lattice |
|
The crystal lattice depicted in Illustration 1 is called a simple cubic lattice. The lattice is a simple pattern of points. In a crystalline solid, these points would be the centers of atoms or molecules.
But how much information do you need to describe the whole lattice? Not much! The simplest repeating pattern necessary to describe a whole crystal is called the unit cell. For the simple cubic lattice in Illustration 1, that's one of the cubes.
Crystals are not usually perfect. Most crystals that exist in nature have defects . In some cases, the defects give the crystals desirable properties. Defects may be holes in the lattice, unaligned planes in the crystal, or other atoms/ions/molecules (impurities) in the lattice.
Sometimes, these defects are undesirable. A good example of this is a flawed diamond. A desirable defect, on the other hand, is a crystal like the ruby. The ruby is aluminum oxide - not a particularly valuable substance in itself. What makes a ruby unique is that some of the aluminum in the crystal is replaced by chromium, giving the ruby its red color.
The properties of solids compared to their classification
How can you tell what the properties of a solid might be without experiment? If you know something about the types of forces that hold it together, you may be able to estimate its properties.
We had four categories of solid when we classified by force. If we rank the forces in order of how difficult it is to break up each force, we would come up with an order like this:
Intermolecular forces < ionic bonds < covalent bonds
Intermolecular forces are easiest to break up. Ionic bonds are harder to break up, and covalent bonds are hardest to break. Metallic bonds vary in strength, so they aren't included above.
Let's correlate the type of solid with each property:
Melting point:
Molecular solids tend to have low melting points.
Ionic solids tend to have high melting points.
Covalent network solids may not melt at all - most decompose before reaching their melting point!
Metallic solids have a wide range of melting points.
Hardness/brittleness:
Molecular solids tend to be soft and brittle.
Ionic solids are hard.
Covalent network solids are among the hardest substances known!
Metallic solids have varying hardness. Many metallic substances are malleable, meaning that they will bend rather than break.
Conductivity:
Molecular solids do not tend to conduct electricity. (They are insulators.)
Ionic solids do not tend to conduct electricity. However, if you melt an ionic solid, the liquid form will conduct electricity.
Covalent network solids do not tend to conduct electricity.
Metallic solids are good conductors of electricity.
If you know a little something about the forces holding a solid together, you can make a good guess about its properties!
Summary
We have discussed solids in some detail, outlining how they are put together - both in terms of the forces that hold the solid together and in terms of the structure of the solid. We have discussed how you would correlate the properties of a solid to what type it is. At this point, you should understand why we have a range of properties in the solid state and what the major differences between solids and the other two phases are.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.