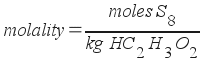
Solve the following problems.
1) A solution is prepared by dissolving 2.15 g of sulfur (S8) in 105.0 g of acetic acid (HC2H3O2). What is the freezing point of the solution?
The freezing point of the solution is 16.31 oC.
|
Freezing point of pure acetic acid: |
16.60 oC |
|
Kf of acetic acid |
3.59 oC/m |
To solve this problem, we must first find the molal concentration of the solution described. Then, we must use the freezing point depression equation.
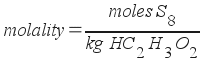
To find molality, we must first find how many moles of S8 are present. We already know the weight of acetic acid.
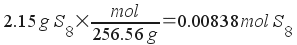
Molality, then, is
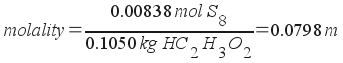
Now, we can use the freezing point depression equation:

So, the freezing point is:
FP = 16.60 oC - 0.287 oC = 16.31 oC
2) An aqueous solution of citric acid (H3C6H5O7) has a concentration of 0.750 m. If the density of the solution is 1.100 g/mL, what is the molar concentration of the solution?
The molar concentration of the solution is 0.721 M.
We have molality (m), and we want molarity (M). Let's compare the two definitions and see what we need to do.
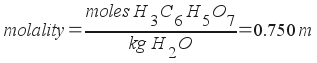
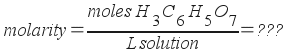
Our task is to find the liters of solution present. Assume you have exactly 1 kilogram of solvent (and thus 0.750 moles of citric acid). Find the weight of this amount of solution, then use the density to change that weight to a volume.
How much does the citric acid in the solution weigh?
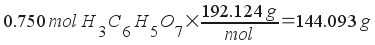
The weight of the solution, then, is the weight of the citric acid plus the weight of the water.
Total solution weight = 1000 g water + 144.093 g citric acid = 1144 g
Now, use the density to find volume:
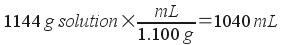
Molarity, then, is simply moles over volume in liters:
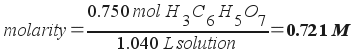
3) Consider the following equilibrium:
N2O4(g) <--> 2NO2(g) ; Kc = 0.125
If you put 0.500 moles of N2O4 in a 10.00 L reaction flask, how many moles of NO2 will be produced at equilibrium? Hint: I am not asking for the concentration of NO 2 as your answer, but you will have to find the concentration to solve the problem. You may need the quadratic formula to solve this problem.
0.538 moles of NO2 gas are produced at equilibrium.
This is an equilibrium problem, so we should write an equilibrium expression:
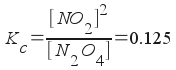
We're asked to find out how much NO2 is produced at equilibrium, so we will have to solve the above for [NO2]. To do that, we must find the initial concentration of N2O4, and express all equilibrium concentrations in terms of one variable.
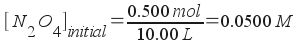
Let's call the amount of N2O4 lost at equilibrium "x" and write out our concentrations at equilibrium:
[N2O4] = 0.0500 - x
[NO2] = 2x
We now plug these into the equilibrium expression:
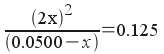
Rearranging, we get:
4x2 + 0.125x - 0.00625 = 0
This is a quadratic equation (a = 4, b = 0.125, c = -0.00625) that we can solve with the quadratic formula. If we do that, we get
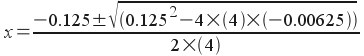
There are two possible solutions:
x = 0.0269
x = -0.0581
We can immediately reject the negative solution. The concentration of NO2 is twice x, and negative solution concentrations don't make physical sense. The other answer seems more reasonable, and we have:
[N2O4] = 0.0500 - x = 0.0231 M
[NO2] = 2x = 0.0538 M
This means that the amount of NO2 present (remembering that the reaction vessel has a 10.00 L volume) is:
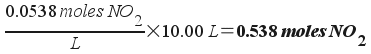
4) Consider the following equilibrium:
COCl2(g) <--> CO(g) + Cl2(g) ; Kc = 8.05x10-4
You have a vessel filled with 1.50 M COCl2, 0.00050 M CO, and 0.00025 M Cl2. Is the reaction at equilibrium? If it is not, will the reaction proceed to form more products or more reactants?
The reaction is not (is/is not) at equilibrium.
The reaction will proceed to produce more products (products/reactants). Answer this one only if the reaction is not at equilibrium!
To answer these questions, find the reaction quotient Qc:
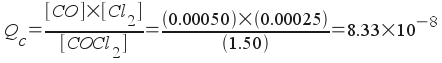
Since Qc is less than Kc, the reaction is not at equilibrium and will proceed to produce more products. The numerator, which represents products, is too small.
All original site content ©2008 Charles Taylor. Page updated: February 04, 2008.