Polarityand Shape
Introduction
The polarity of a substance is related
to many of its important properties - boiling/melting point, what the
substance will dissolve in, etc. Since experimentation is always
costly and sometimes dangerous, we would like to determine the
polarity of a molecule without doing experiments .
Polar bonds?
At first glance, it seems obvious what
you need to have a polar molecule - polar bonds. Polar
bonds are only a part of the requirement, though. For a whole
molecule to be polar, it must have electron density unevenly
distributed across the molecule.
Examples
A good example molecule to study is
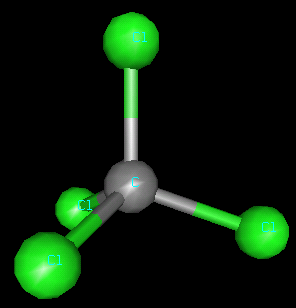
carbon tetrachloride (CCl4). A three dimensional
structure for carbon tetrachloride would look like this.:
|
|
This is a ball-and-stick
model of CCl 4 .
The gray ball is a carbon atom and the green balls are chlorine
atoms.
Carbon tetrachloride is tetrahedral - four things are
around the carbon and all four are atoms.
Carbon-chloride bonds are polar. Look at the
electronegativity difference between
the two atoms.
|
|
Illustration 1: Carbon tetrachloride, a tetrahedral molecule
|
|
We
know that the carbon-chlorine bonds are all polar bonds. There is a
significant electronegativity difference between carbon and chlorine.
So, the chlorine atoms tend to pull electrons towards themselves.
However ,
the four chlorine atoms are all equally spaced around the carbon.
Carbon tetrachloride is symmetric, and the chlorine atoms are
effectively pulling against each other. Carbon tetrachloride, then,
is not a polar
molecule
because there's no net
difference in electron density across the molecule.
Let's look at another example - the water molecule.
|
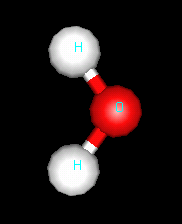
|
The water molecule is bent - it has four things around the
oxygen atom, but only two are other atoms.
Oxygen-hydrogen bonds are polar, since oxygen is much more
electronegative than hydrogen.
|
|
Illustration 2: The bent water molecule
|
|
We know that the hydrogen-oxygen bonds are polar bonds. There is a
significant electronegativity difference between hydrogen and oxygen.
So, the oxygen atom tends to pull electrons towards itself. Since
this molecule is not symmetrical, one side of the molecule
(the oxygen side) takes on a slight negative charge. The side with
the two hydrogen atoms takes on a slight positive charge. Water,
then, is a polar molecule.
|
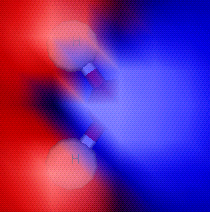
|
This is the same picture as Illustration 2, but
with areas of higher electron density highlighted in blue and
lower electron density highlighted in red.
You can clearly see that one side of the molecule is
electron-deficient, while the other side is electron-rich.
|
|
Illustration 3: Electron density around the water molecule
|
|
Shape is
important! If we'd mistakenly thought water was a linear molecule,
we might have thought water was nonpolar.
If we tried to mix water and carbon tetrachloride, we'd find that
they would not mix. Polar molecules like water tend to associate
with others of their kind than with nonpolar molecules like carbon
tetrachloride.
What makes a polar molecule?
So what makes a polar molecule?
At
least one polar
bond must be present .
(For reference, carbon-hydrogen bonds are considered nonpolar,
since carbon and hydrogen are similar in electronegativity .)
If
there are multiple polar bonds, they
must not be arranged symmetrically.
Some molecules are more polar than
others, just as some bonds are more polar than others. The polarity
of a molecule can be measured, and the dipole moment is often
tabulated for polar molecules. The dipole moment is a
quantitative measure of the polarity of a molecule, expressed in
Debye (D) units. The larger the dipole moment, the more polar
the molecule.
A molecule that is nonpolar
will have a zero dipole moment.
Summary
We have learned
how to tell if a molecule is polar based on the nature of the bonds
in the molecule and the shape of the molecule. In the future,
we will use this information to estimate important properties of
materials.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.


