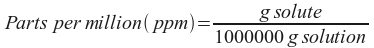
Solubility notes:
Parts per million (ppm): This is a unit of concentration used for very dilute solutions - such as pollutant concentrations or the concentrations of solutions of insoluble salts. It's defined like this:
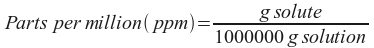
Remember that for pure water (and very dilute solutions in water), 1 g of solution is equivalent to 1 mL. The density of water at room temperature is approximately one gram per milliliter.
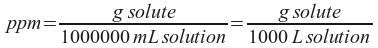
Let's make this unit a little simpler to remember by expressing it in terms of milligrams rather than grams.
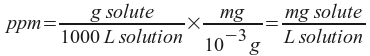
So, for dilute aqueous solutions, parts per million is equivalent to milligrams per liter.
Solubility calculations:
For Ca3(PO4)2, Ksp = 1x10-26. Find the solubility of this salt in mg/L (ppm).
To find the solubility in milligrams per liter (ppm), write a solubility equilibrium and a solubility product constant expression:
Ca3(PO4)2(s) <--> 3Ca2+(aq) + 2PO43-(aq)
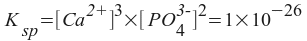
Note that we can solve this if we express the calcium and phosphate ion concentrations in terms of one variable. If we let x equal the amount of calcium phosphate that dissolves, then:
[Ca2+] = 3x
[PO43-] = 2x
and
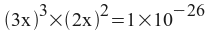
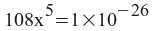
x = 2.4735x10 -6 = moles of Ca 3 (PO 4 ) 2 per liter of solution.
Now all we need to do is convert this number of moles to milligrams:
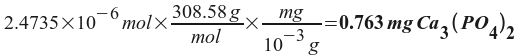
So, the solubility of Ca 3 (PO 4 ) 2 is 0.763 mg/L .
For AgCl, Ksp = 1.8x10-10. Find the solubility of this salt in mg/L (ppm).
To find the solubility in milligrams per liter (ppm), write a solubility equilibrium and a solubility product constant expression:
AgCl(s) <--> Ag+(aq) + Cl-(aq)
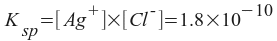
Note that we can solve this if we express the silver and chloride ion concentrations in terms of one variable. If we let x equal the amount of silver chloride that dissolves, then:
[Ag+] = x
[Cl-] = x
and
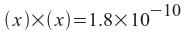
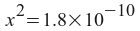
x = 1.34x10 -5 = moles of AgCl per liter of solution.
Now all we need to do is convert this number of moles to milligrams:
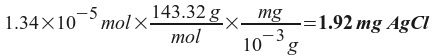
So, the solubility of AgCl is 1.92 mg/L .
The solubility of SrF2 in water is 112 mg/L. Find the Ksp of this salt to two significant digits.
First, we write a solubility equilibrium and a solubility product constant expression:
SrF2(s) <--> Sr2+(aq) + 2F-(aq)
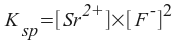
We then express the strontium and fluoride ion concentrations in terms of one variable as if we were going to solve for their concentrations. If we let x equal the amount of strontium fluoride that dissolves, then:
[Sr2+] = x
[F-] = 2x
and
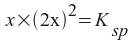
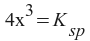
Now, we remember that we defined x as the amount of SrF2 that dissolved - which we know from the problem statement. All we have to do is to convert mg/L to molarity.
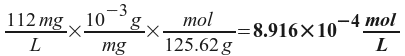
So, x = 2.643x10-5 M. Now, we can find Ksp from the expression we wrote before:
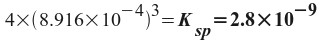
The Ksp of SrF2 is 2.8x10-9.
All original site content ©2008 Charles Taylor. Page updated: April 21, 2008.