Liquid Solid Basics
Introduction
In the previous course, we discussed
the kinetic theory, which
explained how gases are put together. Now, we will discuss
solids and liquids. While we don't have an easy equations like the
ideal gas law that describe solids and liquids, we can
qualitatively discuss liquids and solid properties and how they
follow from the structure and size of their molecules. We
will first quickly review what we know about the three phases of
matter - solid, liquid, and gas. We will then discuss what happens
during a phase transition (the change of a substance from one
phase to another).
The states/phases of matter: Gases
First, since we have discussed gases in
detail, we will begin with a brief review of what we know about
gases.
All gases have much in common. Gases
are:
compressible - You can
change the volume of a gas easily by increasing the pressure you
apply to it.
fluid - A gas will flow
easily from one area to another. Gases flow so easily that
they cannot maintain a definite shape or volume without the
influence of a container.
not dense -
Gases are mostly empty space, with molecules spread far apart in
comparison to the size of the molecules.
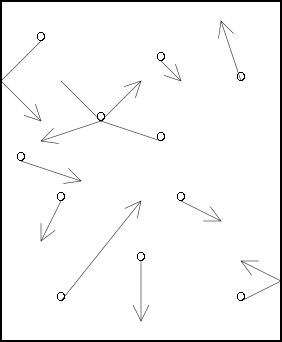
We described gases using the kinetic
theory, as illustrated in the picture below:
|
|
Postulates of kinetic theory, in brief:
Gas molecules are small compared to the empty space
between them.
Gas molecules move in straight lines until they hit
something (another gas molecule or container walls).
There are no forces between the molecules - either
attracting them together or pushing them apart - unless they
collide.
When gas molecules collide, energy may be transferred, but
none is lost as heat.
The temperature is related to the average kinetic energy
(speed) of the molecules.
|
|
Illustration 1 - The kinetic theory of gases
|
|
Kinetic theory leads us to the
conclusion that most gases have very similar physical properties. At
the conditions we're used to seeing gases in, they do. Let's compare
with liquids and solids.
The states/phases of matter: Liquids
What about
liquids? When compared to gases, liquids are:
incompressible - You can't
change the volume of a liquid easily by increasing the pressure you
apply to it.
fluid - A liquid will flow
easily from one area to another and take the shape of the container
they are put in. Liquids do have a definite volume, however.
dense - The molecules in a
liquid are very close together.
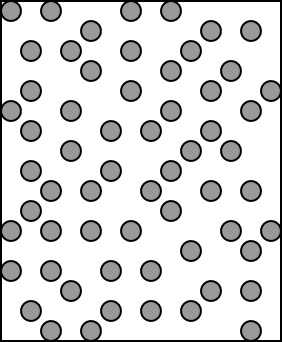
If we were to make a picture of the
liquid phase to compare with the kinetic theory of gases, we might
come up with a picture like this:
|
|
In the liquid state:
Molecules are much closer together than in a gas.
Molecules are free to move around each other (liquids can
flow).
Molecules are held together by what we will call
intermolecular forces
|
|
Illustration 2 - How a liquid is put together
|
|
Liquids
are not as similar in their properties as gases are. It's easy to
see the difference in properties between water and motor oil. This
is due to the fact that the molecules in liquids are close enough to
interact with each another, and that the interactions are different
between different kinds of molecules.
The states/phases of matter: Solids
Solids? When comparing to gases and
liquids, solids are:
incompressible - You can't
change the volume of a solid easily by increasing the pressure you
apply to it. It's even more difficult in most cases to do this to a
solid than a liquid.
rigid - A solid will not
flow easily from one area to another. Solids have definite shape
and volume.
dense - The molecules in a
solid are very close together - usually closer together than in a
liquid.
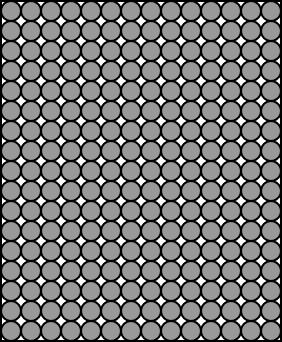
We might graphically depict a solid
like this:
|
|
In the solid state:
Molecules tend to be packed more closely together than in
the liquid or gas state. (Exception: In ice, water molecules are
actually farther apart than in liquid water.)
Molecules can't flow freely around one another. Molecular
motion is limited to vibration.
Molecules are tightly held together by intermolecular
forces.
Most solids have a definite structure - a regular ordering
of molecules called a crystal lattice. This is not true
for all solids.
|
|
Illustration 3 - How a solid is put together
|
|
Like liquids, solids also differ
greatly among themselves. Compare candle wax to a diamond, for
example. These differences are caused by differences in the nature
of the intermolecular forces holding the solids together.
Phase transitions: Going from one
state of matter to another ...
Before we discuss the nature of the
intermolecular forces that make liquids and solids behave
differently from gases, we will first discuss changes between the
various phases of matter. A change from one phase of matter to
another is called a phase transition.
There are six
possible phase transitions listed in the table below
|
Transition name
|
Type
|
Example
|
|
Melting
|
Solid to liquid
|
Ice melts to form liquid water.
|
|
Sublimation
|
Solid to gas
|
Dry ice (frozen CO2) sublimes to form CO2
gas.
|
|
Freezing
|
Liquid to solid
|
Liquid water freezes to form ice.
|
|
Vaporization
|
Liquid to gas
|
When you boil a pot of water, liquid H2O changes to
water vapor (water in the gaseous state).
|
|
Deposition
|
Gas to solid
|
Frost is formed by the deposition of water vapor onto the
ground as ice.
|
|
Condensation
|
Gas to liquid
|
Water will condense onto a cold glass left in a humid room.
(Humid means there is a significant amount of water vapor in the
air.)
|
What happens
during a phase transition?
It
will help us understand the effect of intermolecular forces and
molecular structure on phase changes if we first have an
understanding of what goes on during a phase change (at the
microscopic level). We will discuss a pair of phase changes in some
detail - vaporization/condensation. We'll extend that discussion to
the others.
Vaporization and condensation
Before we explain what's going on in
each of these processes, let's first make an observation.
Vaporization and condensation occur continually over a liquid
surface, no matter the temperature. You don't have to boil water for
it to vaporize, though boiling it speeds the process. At room
temperature, water will evaporate. - liquid water molecules are
vaporized. On the other hand, water vapor condenses on the liquid
surface. A simple piece of evidence for this is the observation that
if you cover the glass of water, the water can't leave the
glass. So the evaporating water must condense back into the liquid
surface.
Why? At given conditions, only so much
of a substance can be in the vapor phase. On the weather report,
they call this the humidity. On a day with 100% humidity, the air is
holding as much water vapor as it can at that temperature. We will
speak of the vapor pressure in CHM 111. The vapor pressure
is the partial pressure of vapor over a liquid surface at equilibrium
(that is, when the rates of evaporation and condensation are equal)
at a given temperature.
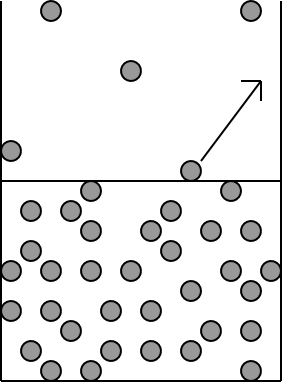
Now - how can we explain vaporization?
Let's look at the kinetic theory of gases and our picture of the
liquid state. For water to get from the liquid to the gas state, the
molecules have to be accelerated to a speed where they can break away
from the other water molecules (and their attractive forces). The
faster the molecule is going, the easier time it has at breaking
away. This would explain more water evaporating at high
temperatures, but how does the water evaporate at low
temperature?
|
|
The more dense liquid state is shown on the bottom.
The horizontal line in the middle of the diagram
represents the liquid surface.
The molecule with the arrow has acquired enough kinetic
energy (speed) to break out of the liquid state and into the gas.
|
|
Illustration 4 - Vaporization
|
|
We can account for vaporization at room
temperatures this way: The definition of temperature, according to
kinetic theory, is the average kinetic energy (speed) of the
molecules. Remember that all molecules are not moving at the
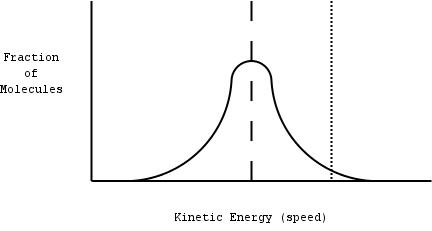
same speed. The distribution of molecules looks something like this:
|
|
This distribution is called a Gaussian in
statistics.
The dashed line represents the average speed (the
temperature).
The dotted line on the right represents the speed
necessary for a molecule to escape into the gas phase.
Note that even at low temperature a fraction of the
molecules are moving fast enough to escape!
A temperature change will shift the curve - lower
temperatures will shift the curve to the left, and higher
temperatures will shift it to the right.
|
|
Illustration 5 - Distribution of molecular speeds at a given
temperature
|
|
So, even at a low temperature, some
molecules still have enough kinetic energy to vaporize. This is how
evaporation at room temperature occurs.
What about boiling? Boiling is a form
of vaporization. But why is there such a thing as a boiling point?
Before you got to chemistry class, you knew that the boiling point
was the temperature at which a substance starts to boil. You know
that water boils at 212oF (or 100oC). If
you've done much cooking , you might know that water boils at a lower
temperature at high altitudes ("high altitude" cooking
instructions account for this). What is boiling to a scientist?
We defined the vapor pressure as
the pressure of the gas phase of a substance over the liquid surface.
We will now define the boiling point of the substance as the
point at which the vapor pressure of the substance equals the
external pressure. When you boil a liquid in an open container, the
external pressure is atmospheric pressure. Let's look at what
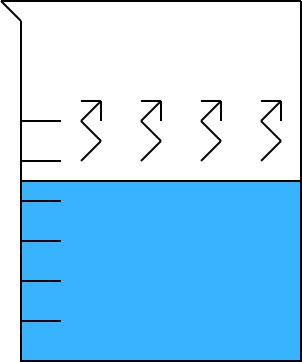
happens when we heat a liquid to boiling. We will use a beaker of
water as an example.
|
|
As we heat the beaker of water, we can observe the following:
The temperature of the water rises.
As the temperature rises, the rate of vaporization
(evaporation) increases.
We may see steam - water vapor - rise from the water
surface.
|
|
Illustration 6 - A beaker of water.
|
|
As we heat the water, its temperature
increases - just as we'd expect. The rate of vaporization also
increases. We start to see steam form when the water gets hot.
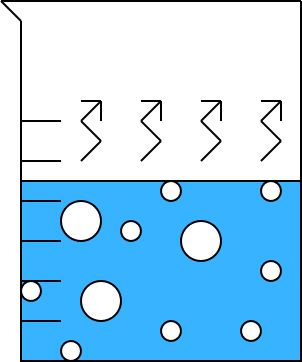
Eventually, the water begins to boil:
|
|
As the water boils:
We must continue to heat the beaker, or else boiling will
stop.
Bubbles of water vapor are visible in the water.
The temperature of the water remains constant. For
pure water at sea level, this is 100oC.
|
|
Illustration 7 - A beaker of boiling water
|
|
When we reach the boiling point, the
vapor pressure of the water is equal to the external pressure. That
means that vapor bubbles can (and do) form in the liquid. These
bubbles that form in the liquid are pure water vapor. Since they're
less dense than the liquid phase, they float to the top and burst,
giving us boiling action.
During the boiling process, the
temperature remains constant. This is an interesting observation!
Why does the temperature remain constant when we are
constantly supplying heat? There is energy involved in the phase
change itself. It requires energy to break up the intermolecular
forces holding the liquid water together. (This energy is called
the heat of vaporization.) So during boiling, the heat added
to the system is used up by the boiling process. The temperature
can't rise again until after boiling is completed and only
water vapor remains .
Thus, boiling is an endothermic
process - it cools the environment. The environment in this case is
the beaker and the remaining water. Since the beaker is being
constantly cooled by the boiling of water, the temperature cannot
rise above the boiling temperature.
This seems counterintuitive at first,
but consider this: Your body cools itself by sweating. The water
secretions don't cool you off directly - they're at the same
temperature your skin is! Sweat doesn't cool you off until it
evaporates. Since the process of evaporation (vaporization) is
endothermic , the
surroundings (your arms and legs, etc.) are cooled. This is
also why you feel hotter on a humid day. On humid days, there is
already a lot of water vapor in the air, so evaporation from
your skin is slowed. You feel warmer because your body can't
cool itself off as fast as it normally would.
How about
condensation? Take our discussion of vaporization and flip it
around. Vaporization requires energy. Condensation releases energy.
When water vapor condenses on the side of a cold glass, the glass is
warmed. The liquid state is a lower energy state than the gas state.
Energy is released when attractive forces begin to hold the
molecules together in the liquid state.
General observations about phase
changes
What happens
during the transition:
In
condensation, freezing, and deposition, intermolecular forces are
formed or strengthened.
In
vaporization, melting, and sublimation, intermolecular forces are
weakened or broken.
Heat:
Processes
that go from a lower energy state to a higher energy one are
endothermic. Examples of these are vaporization, melting,
and sublimation.
Processes
that go from a higher energy state to a lower energy one are
exothermic .
Examples of these are condensation, freezing, and deposition.
Temperature:
During a bulk phase change (a
liquid boiling, a liquid freezing, etc.), the temperature remains
constant. This is so because the phase change itself involves an
energy change. In the case of freezing, the phase change releases
heat, keeping the freezing liquid at a constant temperature. In the
case of boiling, the phase change absorbs heat, keeping the boiling
liquid at a constant temperature.
Summary
In this note pack, we've discussed the
three phases of matter and changes from one phase to another. You
should now have a picture in your mind about how each phase is put
together. You should also be able to visualize what happens during a
phase change and why heat is involved. Remember that we are dealing
with attractive forces between the molecules.
We dealt with vaporization in depth.
Apply the same logic to the other phase changes on your own.
The details are very similar.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.






