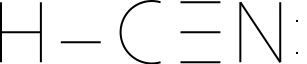
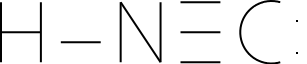
Illustration 1 - Possible structure of hydrogen cyanide
Illustration 2 - Another possible structure of hydrogen cyanide
Introduction
In previous note packs, we discussed how to draw Lewis structures for simple molecules. We have also discussed how to represent delocalized electrons with Lewis structures. Now, we will discuss a method for evaluating how "good" a Lewis structure is compared to other possible Lewis structures for the same compound. We do this by calculating the formal charge of each atom in the compound.
A definition of formal charge
When we speak of formal charges, we're not speaking of charges like the real charge that exists on the sodium ion, Na+, or the chloride ion, Cl-. The formal charge is a hypothetical charge based on two assumptions:
All bonding electrons are shared equally between the bonding atoms.
Lone pairs are not shared with other atoms.
We will use this hypothetical charge to evaluate the Lewis structures we draw.
To find the formal charge on an atom, you can use this simple formula:
|
Formal charge = |
[The number of valence electrons on the original atom ] |
- [The number of bonds connected to the atom] |
- [the number of electrons the atom has in its lone pairs] |
As a check, the sum of all the formal charges of the atoms of a species should equal the charge on the species. For a neutral molecule, this would be zero. For a polyatomic ion, the formal charges would sum up to be the charge of the ion.
An example: Possible structures of hydrogen cyanide
Let's look at an example - the hydrogen cyanide molecule. Hydrogen cyanide contains a hydrogen atom, a carbon atom, and a nitrogen atom. We can draw two possible structures of the hydrogen cyanide molecule. One has a carbon atom in the center, while the other has a nitrogen atom in the center. If we drew these using the method we've previously learned, we would come up with the following structures:
|
|
|
|
Illustration 1 - Possible structure of hydrogen cyanide |
Illustration 2 - Another possible structure of hydrogen cyanide |
The decision we have to make, essentially, is which atom will share all its electrons - carbon or nitrogen. How do we make the decision? We can use an electronegativity argument and say that the atom that is most electronegative (nitrogen, in this case) would share fewer pairs of electrons. We can also calculate the formal charges and use them to make a decision. If we calculated the formal charges of each atom, we would find:
For the HCN structure (C in the middle):
|
Atom |
Sum (valence electrons-bonds-paired electrons) |
Formal Charge |
|---|---|---|
|
H |
1-1-0 |
0 |
|
C |
4-4-0 |
0 |
|
N |
5-3-2 |
0 |
For the HNC structure (N in the middle):
|
Atom |
Sum (valence electrons-bonds-paired electrons) |
Formal Charge |
|---|---|---|
|
H |
1-1-0 |
0 |
|
C |
4-3-2 |
-1 |
|
N |
5-4-0 |
+1 |
We can decide which structure is better following some simple rules of thumb:
The best Lewis structure has formal charges with the lowest magnitude.
If the first rule doesn't let you pick a structure, the best Lewis structure has negative formal charges on the more electronegative atoms of the structure.
Looking at these two rules, we can clearly see that the HCN structure is the best structure. Why? The HCN structure has formal charges of zero, while the HNC structure has +1 and -1 on two atoms. So, by the first rule, HCN is a better structure. The HNC structure also has a -1 charge on carbon while nitrogen has a +1 charge. We've given the more electronegative species (nitrogen) a positive formal charge here. By rule 2, HNC isn't a very good structure.
Experimentally, the hydrogen in the hydrogen cyanide molecule is found bonded with carbon.
Summary
We've discussed how to calculate formal charges and use them to compare several possible Lewis structures. This can get you out of a jam when you're trying to decide which of several possible Lewis structures is best. You can add formal charges to the end of your list of things to check when you draw a Lewis structure.
All original site content ©2007 Charles Taylor. Page updated: November 28, 2007.