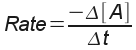 :
the decrease in concentration of reactant A over the change in
time.
:
the decrease in concentration of reactant A over the change in
time.
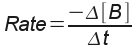 : the decrease in concentration of reactant B over the change in
time.
: the decrease in concentration of reactant B over the change in
time.
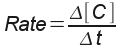 : the increase in concentration of reactant C over the change in
time.
: the increase in concentration of reactant C over the change in
time.
Introduction
We've talked about chemical reactions many, many times in the past. Up to this point, we've mainly talked about the ingredients you need to get a chemical reaction to go. We've talked about how you can take, for example, a solution of sodium chloride and a solution of silver nitrate, mix them together, and end up with silver chloride precipitate. We've talked about how you can take a metal like zinc, react it with an acid like hydrochloric acid, and end up with hydrogen gas as a product. What we haven't discussed up to this point is this: how long does it take for the reaction to run? The study of how fast reactions proceed is called kinetics , and we will now take a look at kinetics and how it applies to the chemistry we've done.
Basic definitions: rates and rate laws
To talk intelligently about kinetics, we first have to learn a little about the language of the field. When we talk about kinetics , we are speaking about reaction rates .
The rate of reaction is the change in the mola r (M) concentration of a reactant or product per unit time. For a generic chemical reaction,
A+ B --> C
... we could express the rate one of three ways:
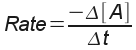 :
the decrease in concentration of reactant A over the change in
time.
:
the decrease in concentration of reactant A over the change in
time.
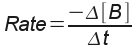 : the decrease in concentration of reactant B over the change in
time.
: the decrease in concentration of reactant B over the change in
time.
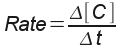 : the increase in concentration of reactant C over the change in
time.
: the increase in concentration of reactant C over the change in
time.
Notes:
[A] means the molar concentration of reactant A. The square brackets can be read as "the molar concentration of". You will see this type of abbreviation throughout the rest of the course.
The Greek letter
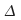 (delta) indicates a change.
(delta) indicates a change.
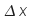 ,
for example, means "the change in x".
,
for example, means "the change in x".
The first two above are rates of disappearance of reactants A and B, while the third is a rate of appearance of product C.
All three of these rates are related by their stoichiometric coefficients.
Mathematically, we express the rate of reaction using the rate law, an equation that expresses the rate of reaction in terms of the concentrations of the species involved in the reaction. For the sample reaction above, the rate law might be written like this:
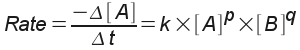
Notes:
We chose the rate we were interested in as the rate of disappearance of reagent A.
These rates from the rate law are instantaneous rates. The rate of reaction changes over the course of the reactions as the concentration of the species in the rate law change.
The exponents "q" and "r" are called reaction orders. In short, the reaction order is how much of an effect the particular reagent has on the speed of the reaction. The order may be positive or negative - a negative reaction order means that the higher the concentration of a reagent, the slower the reaction actually occurs. The most common reaction orders are, however, 0, 1, and 2. If a reagent has an order of zero (a "zeroth order" reaction), then the concentration of that reagent doesn't affect the rate at all - after all, anything raised to the zero power is one.
The "k" is the rate constant. It depends on temperature, but is constant for a given temperature.
These rate laws, as well as the values for reaction order and the rate constant, must usually be determined experimentally. You will do one of these experiments in lab - experiment 14. In this experiment, you will determine the rate of the so-called iodine clock reaction using a stopwatch and an indicator that changes color in the presence of iodine.
Factors that affect the reaction rate
We now need to take some time to discuss factors that influence the reaction rate. After all, our goal as chemists is to be able to predict and control chemical reactions. The speed at which we can produce products is, then, one thing we would really like to have control over.
We will list and then discuss four factors that influence the rate of reaction:
1) The concentration of the reagent: The reaction rate usually increases when we increase the concentration of reactants. This makes logical sense - after all, two molecules must be able to find each other if they are to react, and it's easier for the reacting molecules to find each other if there are more of them together in the same reaction vessel. However, this is not always true. (Remember that the reaction order can be zero or negative).
2) The surface area: This factor is only important if your chemical reaction takes place at some interface between two materials - i.e. where two materials meet each other. For a reaction between a solid and a liquid, for example, you can increase the rate of the reaction by increasing the surface area of the solid - which will increase the amount of liquid in contact with the solid. Rock salt (large crystals of NaCl - low surface area) takes much longer to dissolve in water than table salt (small crystals of NaCl - high surface area) for this reason. If your reaction involves two chemicals in solution, however, there is no interface and surface area is unimportant.
3) The presence of a catalyst: Catalysts are substances that increase the rate of a chemical reaction without being consumed in the reaction. Catalysts are very important in many chemical processes, such as the ones in your body. (Biological catalysts are called enzymes.) We will discuss more about how catalysts work farther down in this note pack.
4) Temperature: Normally, chemical reactions will proceed faster when heated. To explain this assertion (and to explain what catalysts actually do), we need to discuss two related theories on how chemical reactions work.
Collision theory and transition state theory
You probably have a picture in your head already of how chemical reactions might work - and it's probably very close to the picture we're going to discuss here. Collision theory says, simply, that several things must happen for a chemical reaction to occur:
The reactants must collide with each other.
The colliding reactants must hit each other with the proper orientation for a reaction to occur.
The colliding reactants must hit each other with enough energy to initiate the reaction (the "activation energy").
We can graphically illustrate those points like this:
|
|
|
Illustration 1 - A cartoon diagram of collision theory |
In the top part of the cartoon illustration, species A and B hit each other with both the appropriate orientation and speed (energy), so they react, In the middle part, A and B hit each other with enough energy, but with the wrong orientation. The molecules bounce off each other and there is no reaction. In the lower part of the illustration, A and B hit each other, but slowly. They don't have enough energy to react, so there is no reaction.
Since temperature is proportional to the average kinetic energy of the molecules, when we increase temperature, we increase both the sheer number and the energy of the collisions. The reaction rate, then, increases.
We can gain insight into how a catalyst works by looking into transition state theory, a theory that attempts to explain the activation energy - the minimum energy required for two species to react. Transition state theory attempts to explain what happens when species actually react. It says that there is a high-energy state (the transition state) that forms as an intermediate step between reactants and products. This transition state may be an unstable combination of several reactants or it may be an excited (high energy) state of a single molecule. The transition state then breaks down to form products. We can illustrate this with an energy diagram:
|
|
|
|
Illustration 2 - Energy diagram for an endothermic reaction |
|
|
|
|
|
Illustration 3 - Energy diagram for an exothermic reaction. |
|
Note that no matter whether the reaction is exothermic or endothermic, there is still an energy barrier that must be crossed to form products. So, increasing the temperature can increase the rate of an exothermic or an endothermic reaction. This notion explains a lot. If you've ever wondered why flammable materials don't tend to spontaneously burst into flames (this is an exothermic reaction, so the products would have less energy / be more stable than the reactants), it's because of this activation energy barrier. So you need a spark (an input of energy that is large enough to get over the energy barrier) to start a fire.
Arrhenius even put together (before collision theory and transition state theory were formalized) a simple equation from which you could calculate the rate constant k at different temperatures. This simple equation - of course called the Arrhenius equation summarizes (mathematically) collision and transition state theory.
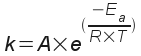
... where
|
k = the rate constant |
R = the ideal gas constant |
|
A = the frequency factor |
T = the absolute temperature |
|
Ea = the activation energy |
|
The frequency factor "A" relates to the fraction of collisions that have the right orientation for the transition state to form."A" and the activation energy Ea are constant for a given chemical reaction. and we can use this equation to estimate k at different temperatures if we are able to find Ea and "A" from measurements. You could do this by measuring rate data at several different temperatures.
Catalysts and transition state theory
But what about catalysts? How do they fit into the picture? We can explain the action of catalysts using the transition state theory. A catalyst speeds up a reaction by "lowering the bar" - that is, it forms a less energetic transition state. This lowers the energy barrier to reaction (and thus speeds up the reaction, since more colliding molecules will have the required energy!). You can depict this on an energy diagram this way:
|
|
|
|
Illustration 4 - An energy diagram of a catalyzed reaction. |
|
The chemical mechanisms of exactly how a catalyst lowers the activation energy range from complex to simple (it depends on what catalyst you're talking about). Some catalysts act to hold reagent molecules at the right orientation so that it's easier to form the transition state. Some catalysts react with one of the reactants, forming a new species that is easier to react with the other reagents. Some cause a completely new transition state to form. The net result is the same, though - faster reactions.
Catalysts need not be in the same phase as the reagents in a chemical reaction. Often, the catalyst will be in a different phase altogether. Take for example the platinum catalyst in your car's catalytic converter. This platinum is in the solid phase, while the reactants coming out of your car's engine are gaseous. This type of catalyst is called a heterogeneous catalyst, while a catalyst that is in the same phase as the reactants (like the copper(II) sulfate solution in experiment 14) is a homogeneous catalyst.
Summary
We have discussed the basic concepts of kinetics. At this point, you should know what is meant by "rate of reaction", and you should be able to look at and use a rate law to predict the speed of a reaction. You should understand what reaction orders and rate constants are and what (if anything) they depend on. You should know the four important factors that can influence the rate of a chemical reaction and how they influence the rate. You should also be familiar with the basic concepts behind collision theory and transition state theory and how they explain the temperature and catalyst effect on the rate of a reaction.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.