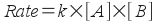
Introduction
So far, we've discussed what equilibrium is and how we can learn things about a reaction (like whether a reaction will proceed to form more reactants or product, the amount of product that will be produced, etc.) by looking at the equilibrium constant. Our ultimate goal as chemists, though, isn't just to know what the reaction will do if left alone. We want to control the reaction - to make it produce a desired amount of product. To control an equilibrium reaction, we need to understand the factors that influence equilibrium.
Le Chateleir's principle
An important thing to note about equilibrium is that at equilibrium, the chemical reaction has not stopped, even though there isn't a net change in the concentration of reactants or products.
A + B <--> C + D
At equilibrium, A and B react to form C and D, while C and D react to form A and B. The rates of these reactions have become equal. If we do something to change the relative rates of the reactions, we will disturb the equilibrium. This will result in the formation of a new equilibrium with different concentrations of A, B, C, and D.
This brings us to Le Chateleir's principle: A system at equilibrium will, when disturbed by any factor, shift to the left of right to counteract that factor. But what are these factors? Factors are things that can change the relative rates of the forward and reverse reactions!
In chapter 14, we defined a rate law as an equation that expresses the rate of reaction in terms of the concentration of reactants or catalysts. A simple rate law for A + B --> C + D (the forward reaction in our equilibrium) might look like:
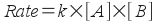
{Note: the actual rate law for the reaction would have to be determined by experiment, but it would contain one or more reactant concentrations}
What you should notice here is that the rate depends on the concentration of reagents! So, if we change the concentration of a reactant or product once equilibrium has been established, we change the relative rates of the forward and reverse reactions and disturb the equilibrium.
A change in concentration of a reactant or product, then, is a factor that disturbs the equilibrium, and according to Le Chateleir's principle, the equilibrium will shift to counteract that factor. How? Let's look at what happens when we vary the concentrations of a mixture of chemicals at equilibrium:
A + B <--> C + D
If you increase [A] (or [B]): The equilibrium will shift to the right, producing more products and consuming reactants. Why? The reactants [A] and [B] appear in the denominator of the equilibrium expression, making the ratio of products to reactants smaller than it would be at equilibrium. The reaction produces more products ("shifts to the right") to reach equilibrium again.
If you decrease [A] (or [B]): The equilibrium will shift to the left, producing more reactants and consuming products. Why? The reactants [A] and [B] appear in the denominator of the equilibrium expression, making the ratio of products to reactants larger than it would be at equilibrium. The reaction produces more reactants ("shifts to the left") to reach equilibrium again.
If you increase [C] (or [D]): The equilibrium will shift to the left, producing more reactants and consuming products. Why? The products [C] and [D] appear in the numerator of the equilibrium expression, making the ratio of products to reactants larger than it would be at equilibrium. The reaction produces more reactants ("shifts to the left") to reach equilibrium again.
If you decrease [C] (or [D]): The equilibrium will shift to the right, producing more products and consuming reactants. Why? The reactants [C] and [D] appear in the numerator of the equilibrium expression, making the ratio of products to reactants smaller than it would be at equilibrium. The reaction produces more products ("shifts to the right") to reach equilibrium again.
How is this useful? If you wanted to increase the amount of product you produce at equilibrium, you can do two things:
Add more reactants to the mixture.
Remove the products as they are formed.
This is particularly useful if the equilibrium doesn't favor products (Kc has a small value). Practically speaking, though, changing the concentrations of reactants and products won't help much if the value of Kc is extremely large or small.
Temperature
We know that temperature affects reaction rates. Temperature also effects equilibrium. You won't see the effect in the equilibrium constant expression, but the effect is buried in Kc (remember, Kc varies with temperature). But why and how is the equilibrium changed by a temperature change?
Remember that reactions can be endothermic (requiring heat to proceed) or exothermic (releasing heat as they proceed). It's helpful to write heat as a reactant or pro duct to see the effect of temperature change on equilibrium:
A + B + heat <--> C + D ; endothermic reaction
By Le Chateleir's principle, the addition of heat to an endothermic reaction (with heat as a "reactant"), would make the equilibrium shift to the right and form more products. You can add heat to a system by increasing its temperature, so:
Endothermic reactions produce more product at higher temperatures.
Endothermic reactions produce less product at lower temperatures.
A + B <--> C + D + heat ; exothermic reaction
Le Chateleir's principle says that the addition of heat to an exothermic reaction (with heat as a "product"), would make the equilibrium shift to the left and form more reactants. You can add heat to a system by increasing its temperature, so:
Exothermic reactions produce less product at higher temperatures.
Exothermic reactions produce more product at lower temperatures.
An interesting thing to note is that all reactions proceed faster at higher temperatures due to the activation energy barrier (see chapter 14 in your textbook and the associated note packs). However, for exothermic reactions, you don't end up getting as much product as you would at a lower temperature. So you get less product, but you get it faster!
Finding the optimum reaction temperature requires that you balance kinetics (reaction rate) and equilibrium.
Pressure
If a reaction involves gases, pressure may influence the equilibrium. Why? Take a look at these representations of a gas at low and high pressure.
|
|
|
|
Illustration 1: A gas at low pressure |
Illustration 2: A gas at high pressure |
The two containers pictured above have the same volume. The container on the left is at a lower pressure than the container on the right. Remember that equilibrium (and reaction rate) depends on concentration. The concentration (the number of moles of gas per liter) is lower when the pressure is lower. This affects the reaction rate and the equilibrium.
When you increase the pressure of a gas by compressing it (reducing its volume), you increase the concentration of the gas. If you increase the pressure by adding an inert gas (one that's not part of the equilibrium), the concentration of the gases in the equilibrium don't change, and the equilibrium is not affected.
When you increase the pressure of a gas-phase reaction by compressing it, you drive the reaction towards whatever side of the reaction produces a smaller number of moles of gas. (This relieves the pressure in the reaction vessel).
For example:
CO(g) + 2 H2(g) <--> CH3OH(g)
If you compressed an equilibrium mixture of carbon monoxide, hydrogen, and methanol, you'd find that the equilibrium would shift to produce more methanol (since only one mole of methanol is produced when a mole of carbon monoxide and two moles of hydrogen are consumed). We'd want to run this reaction at high pressure to maximize our yield of methanol.
Catalysts
Catalysts are chemicals that influence the rate of a chemical reaction by lowering the activation energy. More reactants have the necessary energy to react, so the reaction speeds up. When you lower energy barrier for the forward reaction, you also lower it for the reverse reaction. Equilibrium arrives faster (the reactions are faster), but the concentrations of reactant and product you get are the same as they would have been if you had waited for the equilibrium to form "naturally".
In short, catalysts do not affect the equilibrium mixture. It's good to use a catalyst when the equilibrium constant is favorable, but the reaction itself is slow.
Y ou can get some odd results in a few cases when you use a catalyst but do not then wait for equilibrium to be established (see page 680 in your textbook for an example).
Summary
We've discussed the factors that affect an equilibrium - changes in reactant or product concentration, temperature, and (for gases) pressure. We've also discussed why catalysts do not change the equilibrium. At this point, you should understand what you can do to an equilibrium to make it produce more of what you want it to produce. You should also be able to predict what conditions would be best for running a reaction (high/low temperature, high/low pressure, etc.) given the reaction and information like K and whether the reaction is exothermic or endothermic.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.