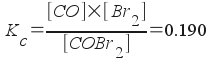
Introduction
We discussed equilibrium in the previous note pack, and you should realize at this point that equilibrium is important to us as chemists. After all, many chemical reactions will proceed to an equilibrium state that is far different from "almost all product and little to no remaining reactant". It's important for us to be able to use equilibrium data (you'll mainly get this as a value for the equilibrium constant Kc) to be able to predict what amount of product we should expect to produce. Industrially, this is important because it affects how chemical processes are designed and, in fact, whether it is economical to produce the product at all. If the equilibrium strongly favors reactants, it might be difficult to cheaply produce enough product to sell. We will learn in this note pack how to work with equilibrium constants to obtain how much product we will obtain for a given equilibrium
A strategy for solving equilibrium problems
We have to start somewhere. We'll first discuss a general procedure for most equilibrium calculations, then we will illustrate the procedure by solving actual equilibrium problems.
First, make sure you have a balanced chemical equation for the equilibrium you're studying. Most "book" problems will give you this in the problem statement in some form. In a real-world application, you might have to use your chemistry knowledge to come up with this.
Next, you will need to write an expression for the equilibrium constant. You can easily write equilibrium constant expressions from the balanced chemical equation. Remember that solids and pure substances do not appear in the equilibrium constant expression by convention (their concentrations do not change) - even if they appear in the reaction.
You will need the value for the equilibrium constant. In textbook problems, this is usually given. In real-world applications, you would look this up in a reference book, or you would perform an experiment to find the value (much like experiment 15B in our lab).
You will typically know the initial concentration of reactant species , but know nothing about any concentration at equilibrium. Since the equilibrium constant expression is valid only at equilibrium, you must write expressions for all equilibrium concentrations using only one variable . This is the part of the process where you'll have to exercise your gray matter and math skills. The easiest way to do this is to arbitrarily set the concentration of one of the products to " x " (you can name this variable anything you want, but you're used to seeing x in math class) . Then, write the other equilibrium concentrations in terms of x using the balanced chemical equation and any information you have about the initial concentration of reactants.
You will now have an expression with one variable, x . You can solve for x using basic algebra techniques. You may have to use the quadratic formula to find x. (Your calculator may be able to solve quadratic equations for you - check your calculator's owner's manual!)
Finally, once you know x , you can find all concentrations at equilibrium . by just plugging your value of x into the expressions you wrote back in step 4. You're done - just make sure you answer what the problem asks (for a "book" problem) or the question your boss asks (for a real-world application). At this point, you should check that your answer makes sense. No concentrations should be negative (that's chemically nonsense!). If your equilibrium constant is large, you should have a significant amount of product. If your equilibrium constant is small, you should have a large amount of reagent left over.
Steps 1-3 simply require you to read the problem and extract the information you need. Steps 4-6 are the actual "work", but you can't do the work if you can't read the problem!
Examples
We can talk at length about how to solve an equilibrium process, but the six step process above won't be clear to you until you actually do some problems. There are sample problems with solutions in your textbook (pages 666-671 in the 7th edition). There are sample problems with solutions in this note pack and in your class notes. Look over these problems. However, it is not enough to just look over problems. You must then do problems on your own without having the full solution in front of you. Practice on the exercises on the above pages in your textbook first, then progress to the odd-numbered problems at the end of the chapter. It's vital that you learn how to solve equilibrium problems now, as the calculations in the next few chapters will be just like these!
Now, let's look at an example problem.
COBr2 can decompose to form carbon monoxide gas and bromine gas via this reaction:
COBr2(g) <--> CO(g) + Br2(g) ; Kc = 0.190
If you start with a 1.00 L vessel containing 0.575 mol COBr2, what is the molar concentration of all three species (COBr2, CO, and Br2) in the vessel at equilibrium?
To solve this, first we note that we have the balanced equation above. We also have a value for the equilibrium constant (0.190). We should write an equilibrium constant expression:
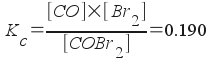
We've now done the preliminary steps [1-3] in our strategy. We're now ready to begin step 4 and solve the problem. We know the initial amount of the reactant . We want to know the initial concentration (in molarity units):
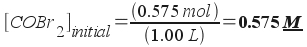
Now we know the initial concentration of the reactant COBr2. Let's set the concentration of bromine, Br2, to be equal to x. (Note- you could have chosen CO just as easily - the solution would be similar). Now we will write all equilibrium concentrations in terms of x :
[Br 2 ] = x
Explanation: We defined x as the concentration of Br 2 .
[CO] = x
Explanation: Every time you produce 1 mole of Br2 molecules, you produce 1 mole of CO molecules; look at the stoichiometric coefficients of Br2 and CO - both 1.
[COBr 2 ] = 0.575 - x
Explanation: Every time you produce 1 Br2, you lose 1 COBr2 ; look at the stoichiometic coefficients of Br2 and COBr2 - both 1. We started with 0.575 M, so the amount remaining at equilibrium is 0.575 - x.
Now, we can write the equilibrium expression in terms of x and solve:
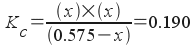
or ...
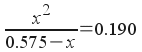
How do we solve this? Realize that with the squared x term, this is a quadratic equation (of the form a x 2 + b x + c = 0) . We'll rearrange the equation above so that it looks like a x 2 + b x + c = 0. To do that, first multiply both sides by (0.575- x ) :
x2 = 0.10925 - 0.575x
Now, simply collect all terms on one side:
x2 + 0.190x - 0.10925 = 0
The equation now has the form a x 2 + b x + c = 0 . We can solve it with the quadratic formula, which says that for an equation of the form a x 2 + b x + c = 0 :
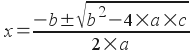
In our equation, a = 1, b = 0.190, and c = -0.10925:
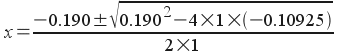
Plugging into the calculator, we get:
x = 0.249 M or x = -0.439 M
The second solution, x = -0.439 M doesn't make any sense chemically (we can't have negative concentrations), so we throw it out.
x = 0.249 M
We can now find the concentrations of all species at equilibrium:
[Br 2 ] = x = 0.249 M
[CO] = x = 0.249 M
[COBr 2 ] = 0.575 - x = 0.326 M
So, we've solved the problem. Since the equilibrium constant is small (0.190 is less than 1), there should be a large amount of reactant remaining, and there is. Our answer makes sense.
Let's look at another example:
Hydrofluoric acid, HF, will break apart in aqueous solution to form hydrogen ions and fluoride ions:
HF(aq) <--> H+(aq) + F-(aq) ; Kc = 6.7x10-4
If you prepare a solution that is 0.5752 M HF, what will the concentration of hydrogen ion be in the solution?
This problem is much like the last one - we know the concentration of the reactant, then are asked to find the concentration of one of the products. Before reading on in this note pack, write down the problem above and try to work it on your own. Now we'll go over the solution:
The equilibrium constant expression is:
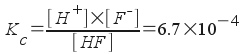
Since we have a balanced equation and we know the initial concentration of reactant species, we now write all concentrations in terms of x :
[H+] = x {this is probably the best choice of x for this problem!}
[F-] = x
[HF] = 0.5752 - x
Now, plug into the equilibrium constant expression:
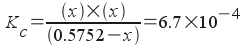
or
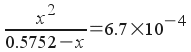
This is a quadratic (note the x2 with no easy way to get rid of it). So, we rearrange the equation to the form a x 2 + b x + c = 0 :
x2 + 0.00067x - 0.000385384 = 0
{Note: I won't round off until the end! }
This is a quadratic with a = 1; b = 0.00067 ; c = -0.000385384 . If you use the quadratic formula, you'll get
x = 0.01930 M or x = -0.01997 M
Since we can't have negative concentrations, we throw out x = -0.01997.
x = 0.01930 M
Since the concentration of H+ = x,
[H+] = 0.01930 M
Note that the concentration of hydrogen ion is very small. This is expected, since the equilibrium constant is also very small!
Summary
In this note pack, we discussed equilibrium calculations and worked some sample problems. We discussed how to start an equilibrium problem - look for a balanced chemical equation and write an equilibrium expression, We then discussed how to write the equilibrium expression in terms of one variable, and then solved the equation using algebra (sometimes requiring the quadratic formula). At this point, you should be able to easily set up the equilibrium problem in terms of a single variable and solve to find the answer. You will need to practice these skills on the suggested problems in this note pack to do well.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.