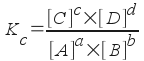
Introduction
We've discussed chemical reactions in some detail up to this point. We've discussed what chemicals you can put together to start a reaction, how fast the reaction occurs, and what can be done to change the rate. We've also discussed - to a point - how to discuss the amount of product produced (called the "yield"). At this point, though, we need to discuss an important point. Not all reactions go to completion, where all the reactants have been consumed and only product remains. Reactions, instead, proceed until they reach equilibrium.
Equilibrium, what is it?
You may have noticed that some reactions do not appear to proceed all the way to completion. Instead, the reaction appears to reach some point where there is some amount of both products and reactants and then stops. What is this state, and what has happened?
Consider a generic reaction:
aA + bB --> cC + dD
... where A, B, C, and D are chemical species and a, b, c, and d are stoichiometric coefficients.
You would, of course, expect this reaction to proceed until A and B are consumed and C and D are formed. However, once C and D are formed, what's to prevent the reaction below from happening?
cC + dD --> aA + bB
The answer is this: the same thing that keeps A and B from becoming C and D - an activation energy barrier. Most reactions can be reversed given sufficient energy. For many reactions, the reverse process requires not too much more energy than the forward process does, and we write these "reversible" reactions this way:
aA + bB <--> cC + dD
The double-headed arrow indicates that the forward process and the reverse process are significant.
So what happens when the reaction just seems to "stop"? The rate of the forward reaction has become equal to the rate of the reverse reaction. When this happens, there is no more net change in the concentrations of either reactants or products. The reaction indeed does appear to cease. (It hasn't actually stopped, though.). This state where the forward and reverse reaction rates are equal is called equilibrium, and will be important to us for much of the rest of the course.
Describing an equilibrium: the equilibrium constant Kc
Remember that the rate of reaction depends on concentrations and temperatures. Equilibrium is established when rates of the forward and reverse reaction are equal, so you might expect that equilibrium, too, has to do with rates and temperatures. For a chemical reaction,
aA + bB <--> cC + dD
we can write the equilibrium constant, Kc, as:
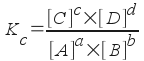
where [A], [B], [C], and [D] are the molar concentrations of each reagent at equilibrium and a, b, c, and d are the stoichiometric coefficients. It is a convention that pure species (solids, pure liquids, etc.) and solvents (water in an aqueous solution) are ignored in equilibrium constant expressions because their molar concentrations don't change.
Note: The "c" subscript in Kc stands for concentration, and Kc is a concentration-based equilibrium constant.
For example, consider the dissolution of solid lead chloride to form lead and chloride ions in water (an equilibrium process - only a very small amount of PbCl2 dissolves in water):
PbCl2(s) <-->Pb2+(aq) + 2 Cl-(aq)
At first glance, the expression for Kc would be
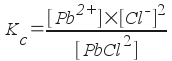
... but since the molar concentration of the pure solid PbCl2 does not change, we write the equilibrium constant expression as:
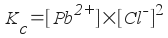
You might wonder at this point where the values of equilibrium constants come from. Like rate constants, they are determined by experiment. We will determine an equilibrium constant in experiment 15B. It's easier to determine an equilibrium constant than a rate constant, but it's difficult to get precise (a large number of significant figures) data. You'll see this when you do the lab.
Equilibrium constants depend (like rate constants) on temperature, but they may increase or decrease (unlike rate constants) with increasing temperature. Whether the equilibrium constant goes up or down with temperature depends on whether the reaction is endothermic or exothermic.
What can you tell about a reaction if you know the equilibrium constant?
We can write equilibrium constant expressions easily enough, and we can obtain the values of the constant Kc by experiment or from books. But what do we have when we have Kc? What can it do for us as chemists? Three of the most important things we can get from Kc are:
Knowledge of whether reactants (the chemicals on the left-hand side of the equation) or products (the chemicals on the right hand side of the equation) are favored when the reaction reaches equilibrium.
Knowledge of whether the reaction will proceed to the left or right if left alone (or if the reaction is already at equilibrium).
The ability to determine the amount of products produced (and reactants left) at equilibrium without doing experiments.
We will discuss the first two of these in this note pack, and we will discuss the third later.
One important thing to note: we can't tell how long it will take to reach equilibrium. Equilibrium constants give you no information about the rate. Equilibrium may be achieved in a millisecond or it may take a week.
What is favored: products or reactants?
This is the easiest thing to determine from the equilibrium constant. No math is required! All you have to do is look at the magnitude of the constant (i.e. how big it is). Remember that the form of the constant is:
aA + bB <--> cC + dD
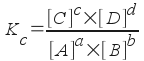
If Kc is large (it's substantially larger then 1), then the formation of products is favored at equilibrium.
If Kc is small (it's substantially smaller than 1), then the formation of reactants is favored at equilibrium.
This is easy to figure out: If Kc is greater than 1, the numerator of the equilibrium constant expression above (the products) is bigger than the denominator (the reactants). If Kc is less than one, the situation is reversed and the denominator (the reactants) of the equilibrium constant expression above is bigger than the numerator (the products).
Which way will the reaction go?
This is a question you might ask yourself if the reaction is one that is slow to reach equilibrium. At some given point, is the reaction going to move forward to make more products or go backwards and form more reactants? You might also want to simply answer the question "Is my reaction at equilibrium yet?"
You can do this by comparing the equilibrium constant to the current state of your chemical reaction. This requires a little math, but it's very simple. All you need to do is to find the reaction quotient, which has the following form.
aA + bB <--> cC + dD
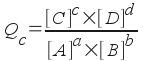
This form should look familiar to you - it's just like the equilibrium constant, except that it uses the current concentrations of reactants and products and not equilibrium concentrations. Once you find the value for Qc (it's a number like Kc), you can compare Qc to Kc to find out something about your reaction. There are three possibilities:
Qc < Kc: If Qc is less than Kc, then the numerator of Qc (which represents products) is smaller than it would be at equilibrium. The reaction, then, proceeds to the right to form more products.
Qc = Kc: If Qc equals Kc, then the reaction is at equilibrium and the amounts of reactants and products will remain the same.
Qc > Kc: If Qc is larger than Kc, then the numerator of Qc (which represents products) is larger than it would be at equilibrium. The reaction, then, proceeds to the left to form more reactants (and eliminate "excess" product).
A sample application of K c
As an example of how to use Kc, let's consider the following equilibrium:
2 H2 + S2 <--> 2 H2S , where Kc = 1.08x107
First, what does the simple value of Kc tell us? Kc is much greater than 1, so we should expect the product to dominate at equilibrium.
If we have a mixture where each chemical involved in the equilibrium was present at a concentration of 2.5 M, which direction will the reaction proceed?
To answer this question, we find Q c (the reaction quotient) and compare it to K c (the equilibrium constant). The form of Qc for this reaction is:
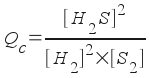
Plugging in the concentrations, we get:
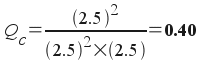
Since 0.40 < 1.08x107, we know that this reaction mixture is not at equilibrium and the reaction will proceed to the right to produce more products.
Gas-phase reactions
In some cases, it is easier to deal with gas-phase chemical reactions in terms of the partial pressures (rather than concentrations) of reactants and products. You can write an expression for the equilibrium of a gas-phase reaction that looks like this:
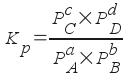
where PA - PD are the partial pressures of each gas and a-c are stoichiometric coefficients.
This equilibrium constant is pressure-based and has a different numerical value than the concentration-based constant K c . Despite the difference, you can use a pressure-based equilibrium constant in the same way that you'd use a concentration-based constant. You just have to use pressures rather than concentrations.
Summary
In this note pack, we've learned what equilibrium and the equilibrium constant are. We've also learned how to write an expression for the equilibrium constant and how to use the equilibrium constant to predict two things about a chemical reaction: whether products or reactants dominate at equilibrium and whether a reaction will proceed to the left or right from its current state. We also noted that you can actually use KC to find out equilibrium concentrations, but that will be the topic of a future note pack.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.