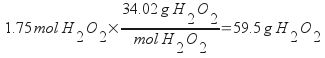
1) What is the mass percentage of H 2 O 2 in a solution that is 1.75 m H 2 O 2 (the solvent is water)?
1.75 m H2O2 is 5.62% H2O2 by mass.
Solution
This problem is easy to solve if you realize that molality is based on the mass of the solvent. Assume a basis of exactly one kilogram of solvent to solve this problem. In one kilogram of H2O, there are 1.75 moles of H2O2. Convert the moles of H2O2 to grams.
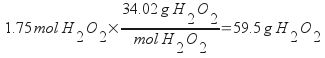
Divide by the total mass of the solution (add the water and the peroxide) and multiply by 100% to get the mass percentage.
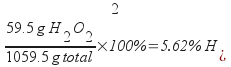
2) A 12.5 M H2SO4 solution has a density of 1.66 g/mL. What is the molality of the solution? The solvent is water.
12.5 M H2SO4 is 28.8 m H2SO4.
Solution
Since you are starting with a unit based on the volume of solution, assume a basis of exactly one liter of solution to solve this problem. You will need to calculate the mass of water (the solvent) present. To do that, you will first need to determine the total mass of a liter of solution, then the mass of sulfuric acid present.
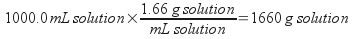
Now, find the mass of sulfuric acid present.
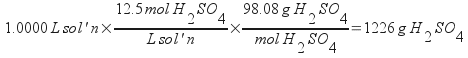
Subtract these to find the mass of water: 434 g, or 0.434 kg. Once the mass of water is known, then simply use the definition of molality. You already know how many moles of sulfuric acid are present.
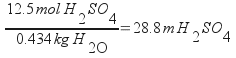
3) How much potassium cyanide (KCN) would you need to weigh our to make 500.0 mL of a 0.275 M potassium cyanide solution?
Weigh out 8.95 g of KCN.
Solution
To solve this problem, find out how much of the 500.0 mL of solution is potassium cyanide, then convert moles to mass.
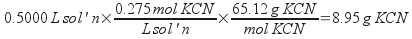
4) How do you prepare 555 grams of an aqueous solution of KI that is 7.6% potassium iodide (KI) by mass?
Dissolve 42 g KI in 513 g water.
Solution
Use the percentage to figure out the mass of potassium iodide in 555 g solution.
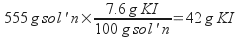
Subtract this mass from the total mass to find the mass of water: 513 g .
5) If a 150.0 g sample of rubbing alcohol (a solution of isopropanol, C3H7OH, in water) contains 97.0 g isopropanol, calculate the mole fraction of each component of the sample, the mass percentage of isopropanol in the sample, and the molality of isopropanol in the sample.
The mole fraction of isopropanol in the sample is 0.353.
The mole fraction of water in the sample is 0.646.
The mass percentage of isopropanol in the sample is 64.7%.
The molality of the isopropanol in the sample is 30.4 m.
Solution
This problem asks you to calculate several different things based on the same information, so you'll have to assemble some information together to answer all the questions. You will need the mass of water present to determine both the mole fractions and the molality. You will also need to calculate the moles of isopropanol (for mole fraction and molality) and water (for mole fraction) present. The problem statement already contains enough information to directly calculate the mass percent.
Subtracting the mass of isopropanol from the total sample mass gives the mass of water; 53.0 g. Next, calculate the moles of both isopropanol and water.
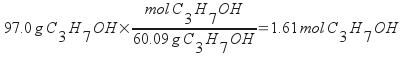
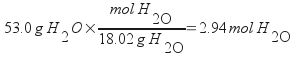
Calculate the mole fractions by dividing each of these by the total number of moles present in the sample.
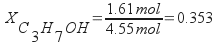
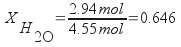
Calculate the mass percentage of isopropanol from the mass of isopropanol and the total mass of the sample.
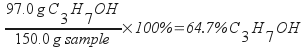
Calculate molality from the moles of isopropanol and mass of water.
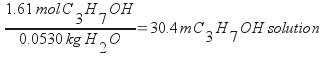
All original site content ©2008 Charles Taylor. Page updated: February 04, 2008.