
Introduction
Weak acids and bases do not completely ionize in solution. It is sometimes useful to talk about how much of a weak acid or base reacts with water to form ions. To do this, we use the degree of ionization and the percent ionization. Both of these may be calculated from experimental data or from the acid or base ionization constants.
Definitions
The degree of ionization is defined as the fraction of a weak acid or base which ionizes. It can be calculated this way.

The percent ionization is just the degree of ionization expressed as a percentage.
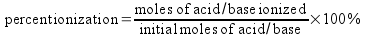
You can find the moles ionized either experimentally or from an equilibrium calculation, while you know the initial moles of acid/base if you know the initial concentration of the solution.
Trends
As the acid or base becomes more dilute, the degree of ionization increases. Why? Let's look at it this way. Let's say that we have a solution of acetic acid at equilibrium.
HC2H3O2(aq) + H2O(l) <--> H3O+(aq) + C2H3O2-(aq)
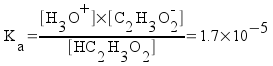
Now, add enough water to half the concentration of all species present.
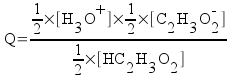
Do a little rearrangement of the equation.
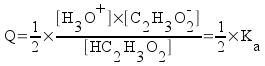
So, if Q is less than Ka, then the reaction will proceed to the right and produce more products (ions, for an acid or base ionization reaction). Therefore the degree of ionization will increase as we dilute a weak acid or base. We can also show that the degree of ionization decreases as the concentration of acid or base increases.
Calculating degree of ionization with an equilibrium calculation
Calculating the degree of ionization using the acid or base ionization constant is really no different than most other acid/base equilibrium calculations. To illustrate this, we will calculate the degree of ionization for two concentrations of acetic acid solution.
Calculate the degree of ionization and percent ionization for 0.020 M acetic acid and 0.20 M acetic acid.
The equilibrium and Ka expression for acetic acid in water look like this.
HC2H3O2(aq) + H2O(l) <--> H3O+(aq) + C2H3O2-(aq)
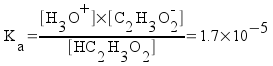
For the 0.020 M acetic acid,
[HC2H3O2] = 0.020 - x
[H3O+] = x
[C2H3O2-] = x
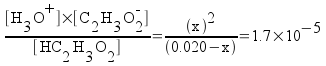
Rearrange the equation.
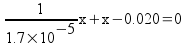
This is a quadratic, with a = 1/1,7x10-5, b = 1, c = -0.020.
x = 0.000574 (ignoring the negative solution)
Since x is equal to the change in concentration of the acid, it's also equal to the number of moles of acid dissociated per liter of acid. The initial moles of acid per liter is equal to the original acid concentration. We can calculate degree of ionization now.
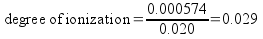
The percent ionization, then, is 2.9%..
For the 0.20 M acetic acid,
[HC2H3O2] = 0.20 - x
[H3O+] = x
[C2H3O2-] = x
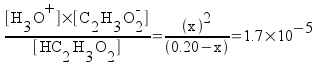
Rearrange the equation.
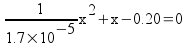
This is a quadratic, with a = 1/1,7x10-5, b = 1, c = -0.20.
x = 0.00184 (ignoring the negative solution)
Since x is equal to the change in concentration of the acid, it's also equal to the number of moles of acid dissociated per liter of acid. The initial moles of acid per liter is equal to the original acid concentration. We can calculate degree of ionization now.
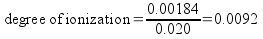
The percent ionization, then, is 0.92%..
[You could have solved these problems by assuming that x was negligible compared to the initial concentration. Try it that way and verify that you get the same answers!]
Notice that the degree of ionization is much less in the more concentrated acid, though there are more actual ions present in the more concentrated solution.
Summary
After reading this note pack, you should be familiar with the degree of ionization and the percent ionization and how to calculate them from an equilibrium calculation. You should also be aware that the degree of ionization increases as concentration goes up, and decreases as concentration goes down.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.