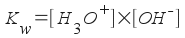
Introduction
If you've watched any television over the past few years, you've heard of pH. You probably already know a fair bit about it. Now that we understand equilibrium, we can appreciate what the pH scale actually tells us. In this note pack, we will discuss the equilibrium that gives rise to the pH scale, as well as the effect of strong acids and bases on this equilibrium.
The water equilibrium
You might have noticed in our previous discussions that water can act as an acid or a base, depending on the situation. Water can actually react with itself in an equilibrium reaction (the process is called self-ionization ) :
H2O+ H2O <--> H3O+ + OH-
OR (in short form):
H2O <--> H+ + OH-
The equilibrium constant expression for this reaction is, then:
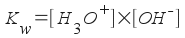
[Note: "w" stands for water]
Experimentally, it's been found that Kw = 1.0x10-14. This number is much smaller than 1, so we expect the equilibrium to strongly favor the water molecules rather than the ions. In fact, you can easily calculate the concentration of hydronium ions and hydroxide ions in water. How?
We can see from the equation that in pure water, whenever a hydronium ion is produced, a hydroxide ion is produced. So, the concentration of hydronium ion and hydroxide ion are equal. Call these concentrations "x", and solve.
x2 = 1.0x10-14
x = 1.0x10-7 M
Only a small amount of hydronium and hydroxide ions are produced, but these can be measured by devices like pH meters and indicators like litmus or phenolphthalein.
pH - what is it?
Speaking of pH meters, we need to discuss what pH is in order for you to make sense of the numbers you'd get from a pH meter. The "p" is a mathematical function. It simply means "take the negative logarithm of". pH, then, is defined as the negative logarithm of the hydronium (or hydrogen) ion concentration. You may also see pOH, which is the negative logarithm of the hydroxide ion concentration. Some useful formulas:
pH = -log([H+])
[H+] = 10-pH
[Note: This logarithm is the log base 10, which is usually found on calculators as "log" or "log10". This is not the natural logarithm (log base e), which is usually found on calculators as "ln".]
Why do we bother with this notation? Because it's cumbersome to deal with numbers like "1.0x10-7 M". They're hard for us to talk about and wrap our minds around. So, we use pH. If a solution contains 1.0x10-7 M hydronium ion, it has a pH of 7.00.
The addition of acids and bases to water affect the equilibrium of H3O+ and OH- and thus affect the pH. We can see how acidic or basic a solution is by measuring pH. We get a scale that looks something like the illustration below.
|
|
|
Illustration 1 - The pH scale |
Solutions with a pH of less than 7 (which means their [H3O+] is greater than 1.0x10-7 M) are considered acidic. These solutions contain less than 1.0x10-7 M hydroxide.
Solutions with a pH of greater than 7 (which means their [H3O+] is less than 1.0x10-7 M) are considered basic. These solutions contain more than 1.0x10-7 M hydroxide.
Solutions whose pH is equal to 7 are considered neutral. They contain exactly 1.0x10-7 M of both hydronium ion and hydroxide ion.
One thing you can tell quickly about an unknown aqueous solution is whether it's acidic, basic, or neutral. All you need is a pH meter or some pH indicator paper. You might need to know this to know whether it's safe to mix the solution with another or to determine how to dispose of the solution.
The affect of strong acids and bases on the water equilibrium (and pH!)
The presence of acids and
bases affect the water equilibrium. As you can see from the
equilibrium expression,
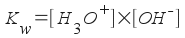 ,
if you change the concentration of H3O+, the
concentration of OH- will change to maintain the
equilibrium.
,
if you change the concentration of H3O+, the
concentration of OH- will change to maintain the
equilibrium.
In the case of strong acids and bases, the effect on the pH is very easy to determine. Strong acids and bases completely ionize in water to give hydronium ions and hydroxide ions, respectively. Let's look at the case of a solution of a strong acid:
What is the pH of a 0.0025 M HCl solution?
HCl reacts with water in this way:
HCl + H 2 O --> H 3 O + + Cl -
Since HCl is a strong acid, this reaction goes to completion, and for every formula unit of HCl present, a H3O+ ion is formed. Since we started off with 0.0025 M HCl, we now have 0.0025 M H3O+.
If [H3O+] = 0.0025 M, then the pH = -log(0.0025). The pH of the solution is 2.60.
[Note: What about the hydronium ion produced by the self-ionization of water? We have 0.0025 M hydronium ion from the acid, while pure water can only manage to form 1.0x10-7 M hydronium ion. Water's contribution of hydronium ion is so small we can neglect it without affecting our results.]
What about a solution of a strong base?
What is the pH of a 0.0025 M NaOH solution?
Since NaOH is a strong base, it completely dissociates in water:
NaOH --> Na+ + OH-
As before, this reaction goes to completion. For every formula unit of NaOH present, a hydroxide ion is formed. Since we started off with 0.0025 M NaOH , we now have 0.0025 M OH-. (As before, the contribution to the hydroxide concentration by water's self ionization is negligible.)
Therefore, [OH-] = 0.0025 M. However, we're not asked about the hydroxide concentration. We're asked about the hydronium ion concentration (that's what you use to find pH!). We can relate hydronium ion concentration to hydroxide ion concentration in any aqueous solution by using the water equilibrium:
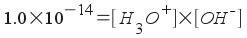
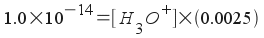
Solving, we find that [H3O+] = 4.00x10-12 M. The pH of the solution is 11.40.
Do these numbers make sense? Yes. Here is a table that shows you what you should expect in an acidic or basic solution.
|
Solution type |
pH |
[H3O+] |
[OH-] |
|---|---|---|---|
|
Acidic |
less than 7 |
more than 1.0x10-7 |
less than 1.0x10-7 |
|
Basic |
more than 7 |
less than 1.0x10-7 |
more than 1.0x10-7 |
Summary
In this note pack, we've discussed the water equilibrium and the pH scale. You should be able to find the pH of an aqueous solution given the concentration of hydronium or hydroxide ions. You should be able to tell whether a solution is acidic, basic, or neutral if you know its pH. We also discussed the affect of strong acids or strong bases on pH. You should be able to calculate the pH of a strong acid solution or a strong base solution.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.