Illustration 1 - A generic Lewis acid-base reaction
Introduction
You probably are already familiar with acids and bases in some way. In CHM 110, you learned that acids and bases react in what are called neutralization reactions, usually forming an ionic salt and water. You learned a simple definition of acids and bases. Now that we understand equilibrium, though, we are ready to understand what acids and bases really are - and what this number called the pH has to do with them.
Three definitions of acids and bases
Science constantly revises itself. Often, a scientist will come up with a workable theory that explains the facts as he/she knows them. Later, new facts emerge that the current theory can't explain (or simply doesn't apply to). The theory will either be revised to handle the new facts or scrapped for a new theory which can handle both the old and new facts. You've seen this already in the beginning of CHM 110 with Dalton's atomic theory, the planetary model of the atom, etc. You're going to see it again now with the definitions of acids and bases.
Arrhenius definition
The first definition of acids and bases we will discuss is the oldest (1884). It's also the simplest and the one you probably already know - whether from CHM 110 or from a middle or high school physical sciences course: the Arrhenius definition.
Arrhenius defined an acid as a substance that increased the concentration of hydronium ions, [H3O+], when dissolved in water. [Note: We usually write these as if they were bare protons, H+, in water. This is done to simplify some equations and equilibrium expressions.]
A classic example of an Arrhenius acid is hydrochloric acid, HCl. When put in water, HCl dissociates (breaks apart) to form hydronium ions and chloride ions. We can write the reaction like this:
HCl(aq) + H2O(l) <--> H3O+(aq) + Cl-(aq)
OR (in short form):
HCl(aq) <--> H+(aq) + Cl-(aq)
Hydrochloric acid increases [H3O+] in the water, so it is an acid by the Arrhenius definition.
Arrhenius defined an base as a substance that increased the concentration of hydroxide ions, [OH-], when dissolved in water.
The classic example of an Arrhenius base is sodium hydroxide, NaOH. This is a soluble ionic compound. When dissolved in water, NaOH dissociates to form sodium ions and hydroxide ions. We can write the reaction like this:
NaOH(aq) <--> Na+(aq) + OH-(aq)
Sodium hydroxide increases [OH-] in the water, so it is a base by the Arrhenius definition.
An acid-base reaction, then, is really the reaction of the hydronium (hydrogen) ion from the acid with the hydroxide ion from the base:
H3O+(aq) + OH-(aq) <--> 2 H2O(l)
OR (in short form):
H+(aq) + OH-(aq) <--> H2O(l)
Note that water is a product of the reaction (no matter how you write it). A reaction of an Arrhenius acid with an Arrhenius base will produce water. Let's look at the reaction of our example acid with our example base:
HCl(aq) + NaOH(aq) <--> H2O(l) + NaCl(aq)
Remember that HCl in water produces hydronium (hydrogen) ion and chloride ion, while NaOH in water produces sodium ion and hydroxide ion. The hydronium (hydrogen) and hydroxide ions are the source of the water, while the sodium and chloride ions are the source of the sodium chloride.
Note: The reaction between hydrochloric acid and sodium hydroxide is called a neutralization reaction. You can also think of it as a double displacement reaction, where water is produced as the stable product.
Bronsted-Lowry definition
You might have already noticed a primary limitation of the Arrhenius definition - it only applies to aqueous solutions. If your substances haven't been dissolved in water, the Arrhenius definition simply doesn't apply. Since we sometimes wish to study substances that are not dissolved in water, we need a broader definition for acids and bases. Notice that in the reaction between the Arrhenius acid and the Arrhenius base above, the acid gives up a proton (H+) to the base. Scientists noticed this, and redefined an acid-base reaction as a proton transfer between the acid and base. The definitions of acids and bases themselves were also revised.
A Bronsted-Lowry acid is a substance that is able to donate a proton to another substance . Let's look at an example - hydrochloric acid again:
HCl(aq) + H2O(l) <--> H3O+(aq) + Cl-(aq)
Note that the hydrochloric acid donates a proton (a hydrogen ion) to the water molecule . Since hydrochloric acid donates a proton, it is an acid by the Bronsted-Lowry definition.
A Bronsted-Lowry base is a substance that is able to accept a proton from another substance . Let's look at an example - ammonia, NH 3 :
NH 3 ( aq) + H2O(l) <--> NH4+(aq) + OH-(aq)
Note that the ammonia molecule accepts a proton (a hydrogen ion) from the water molecule, becoming the ammonium ion, NH 4 + . Since ammonia accepts a proton, it is a base by the Bronsted-Lowry definition.
Another thing to note here is the role of water. In the reaction with ammonia, water is a Bronsted-Lowry acid because it donates a proton. In the reaction with hydrochloric acid, water is a Bronsted-Lowry base. Depending on what it reacts with, water can act as an acid or a base!
This reaction is an equilibrium reaction -it can proceed either to the left or the right. We can label all of the substances above as acids or bases. NH 3 is, of course, a base. H 2 O is, here, an acid. NH 4 + is also an acid - it can donate a proton to OH - in the reverse reaction. OH - is, then, a base, as it can accept a proton.
Notice that an acid on one side of the reaction is closely related to a base on the other side of the reaction. They differ by a proton. These related acids and bases are called conjugate pairs. Conjugate pairs are two substances (an acid and a base) that differ only by the addition or removal of a proton. Here are some examples:
|
Substance |
Conjugate |
|---|---|
|
NH3 (base) |
NH4+ (conjugate acid) |
|
H2O (acid) |
OH- (conjugate base) |
|
H2O (base) |
H3O+ (conjugate acid) |
|
HCl (acid) |
Cl- (conjugate base) |
Here's the general form of an acid-base reaction according to the Bronsted-Lowry definition:
|
HA |
+ |
B |
<--> |
BH+ |
+ |
A- |
|
(acid) |
+ |
(base) |
<--> |
(conjugate acid) |
+ |
(conjugate base) |
See if you can label the acids and bases in the following reaction:
HNO2(aq) + (CH3)2NH(aq) <--> (CH3)2NH2+(aq) + NO2-(aq)
Answers:
HNO2 is an acid, NO2- is its conjugate base.
(CH3)2NH is a base, (CH3)2NH2+ is its conjugate acid.
See if you can write the products of the following reaction:
HNO 3 (aq) + CH 3 NH 2 (aq) <-->
[Hint: CH3NH2 is chemically similar to ammonia and is a Bronsted-Lowry base]
Answer:
The products are NO3- and CH3NH3+
Lewis definition
The Bronsted-Lowry definition is quite useful and, if you were to become an analytical chemist, the one you would use most often. However, some substances that don't either donate or accept hydrogen ions appear to have acidic or basic properties. In these situations, the Arrhenius definition and the Bronsted-Lowry definition are inadequate. A new view of acid-base reactions was proposed. Acid-base chemistry could be explained in terms of electron transfer. This is called the Lewis definition of acids and bases.
A Lewis acid is a substance that is able to accept a pair of electrons from another substance, while a Lewis base is a substance that can donate a pair of electrons to another substance.
[Note: Don't get this definition and the Bronsted-Lowry definition confused!]
The general form of a Lewis acid-base reaction would look like this:
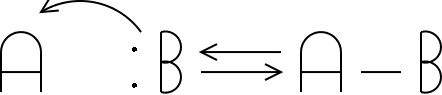
A + :B <--> A-B
A is the Lewis acid, and B is the Lewis base. I've shown the pair of electrons on the Lewis base above. A Lewis base needs unbonded electrons to donate to another atom. If you draw a Lewis structure, you'll see these electrons as lone pairs.
What's happened in this reaction? Electrons were transferred from the base to the acid.
|
|
|
Illustration 1 - A generic Lewis acid-base reaction |
Note that a bond was formed between A (the acid) and B (the base). This bond is a covalent bond formed by the sharing of the donated electrons between A and B. Let's look at some other Lewis acid-base reactions.
Iron(III), Fe3+, can react with thiocyanate ion, SCN-, to produce a complex ion, Fe(SCN)2+, containing iron bonded to thiocyanate ion. We've seen this deep red complex in several lab experiments, but you probably didn't know that this chemistry is actually classified as an acid-base reaction! Let's look at it.
Fe3+(aq) + SCN-(aq) <--> Fe(SCN)2+
How is this an acid-base reaction? The answer is that the thiocyanate ion can donate a pair of electrons. The Lewis structure of thiocyanate ion looks like this
|
|
|
Illustration 2 - The Lewis structure of thiocyanate ion |
SCN- ion has several lone pairs of electrons (and can act as a Lewis base). Iron(III) ion is the Lewis acid. Transition metal ions frequently react with Lewis bases to form complex ions.
Let's look at a more familiar acid-base reaction: ammonia and water. How is this an acid-base reaction by the Lewis definition? The reaction is:
NH3(aq) + H2O(l) <--> NH4+(aq) + OH-(aq)
We know ammonia is a Bronsted-Lowry base, as it accepts a proton. Is it a Lewis base? Let's look at the structure.
|
|
|
|
Illustration 3 - The structure of ammonia |
|
Ammonia, because of its lone pair, can act as a Lewis base. The ammonia shares its lone pair with a hydrogen from the water molecule - forming a bond with the hydrogen. At the same time, the bond between that hydrogen and the oxygen in water breaks - making the products ammonium ion and hydroxide ion. Ammonia is a Lewis base, and the water is the Lewis acid.
The Lewis definition will be important to you if you should go into organic chemistry, as many organic reactions can be described as Lewis acid-base reactions. The formation of complexes (or complex ions) also involves Lewis acid-base chemistry , as we have shown above.
An important note
You should realize that the Bronsted-Lowry definition is an "improvement" on the Arrhenius definition, and the Lewis defintion is an "improvement" on the Bronsted-Lowry defintion. Each definition is broader than the one before. So:
If you know something is an Arrhenius acid, you know it is also a Bronsted-Lowry acid and a Lewis acid.
If you know something is a Bronsted-Lowry acid, you know it is also a Lewis acid.
If you know something is an Arrhenius base, you know it is also a Bronsted-Lowry base and a Lewis base.
If you know something is a Bronsted-Lowry base, you know it is also a Lewis base.
Unless you're an organic chemist, the definition you will usually use is the Bronsted-Lowry definition!
Summary
We have discussed the three most useful definitions of acids and bases: Arrhenius, Bronsted-Lowry, and Lewis. You should know all three of these and be able to identify acids and bases in chemical reactions. In addition, you should be able to predict the products of acid-base reactions. In the next note pack, we will discuss what is meant by "strong" and "weak" acids and bases.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.