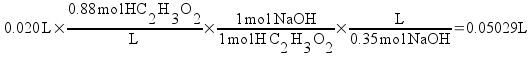
Introduction
Volumetric analysis, usually called titration , is an important tool in real-world chemistry labs. Titration allows you to determine the concentration of an unknown solution by reacting it with a solution whose concentration is known. You can then calculate the concentration of the original solution using stoichiometry.
In your first chemistry course, you probably performed a few simple titration experiments. The common beginner's experiment is the titration of a vinegar solution (5% acetic acid) with sodium hydroxide. This is an acid-base titration or a neutralization titration, and now that you understand something about equilibrium, you are now ready to understand what's going on chemically during one of these titrations.
In this note pack, we will examine titrations of weak acids with strong bases, and titrations of weak bases with strong acids.
Monitoring an acid-base titration: The titration of vinegar with sodium hydroxide
The titration of vinegar with sodium hydroxide uses this chemical reaction.
HC2H3O2(aq) + NaOH(aq) <--> NaC2H3O2(aq) + H2O(l)
For the titration to give you useful results, you need to know when you have added the amount of sodium hydroxide that is exactly enough to react away all of the acetic acid in the vinegar.
Usually, the titration of vinegar solutions is performed using phenolphthalein (a weak acid) as indicator. This indicator changes from its colorless acid form to its pink conjugate base across a pH range from 8 to 10. You are told that when the phenolphthalein changes from clear to pink, you have added the right amount of sodium hydroxide to just react away the acetic acid. But how do you really know?
You can follow the progress of the titration another way. You know that the pH of the vinegar solution will start off acidic, then will increase as hydroxide as added. So, what would you see if you monitored the pH of a vinegar-sodium hydroxide titration?
Vinegar is usually about 0.88 M acetic acid. Let's assume that we take 20 mL of vinegar and titrate it with 0.35 M sodium hydroxide solution. We can calculate the equivalence point (the point where the exact amount of base required to neutralize the acid) easily.
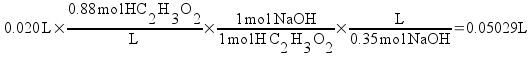
So, it takes 50.29 mL of base to reach the equivalence point. Keeping that in mind, let's look at the pH of the titration as we add base. You can do this experimentally by titrating while measuring the pH of the solution with a pH meter.
|
|
|
Illustration 1 - pH of vinegar titration vs. amount of NaOH solution added |
The plot shows three main features. The equivalence point of the titration is marked by a steep pH rise. Before the equivalence point, there is a region called the buffer region where the pH remains fairly steady. After the equivalence point, there is a region where pH levels out again.
The pH at the equivalence point is shown on the plot with a dotted line,
The buffer region
From about 10 mL of base added to about 45 mL of base added. we are in the buffer region, where the addition of strong base does not change the pH very much. Remember that buffer solutions are made from weak acids/bases mixed with their conjugates, and that's exactly what we have here. The strong base sodium hydroxide reacts with acetic acid to produce the conjugate base of acetic acid - acetate ion. The pH here is controlled by the acetic acid / acetate ion buffer that is being made by the titration, and can be calculated using the Henderson-Hasselbalch equation.
The equivalence point
As we approach the equivalence point, we see that the pH begins to climb very steeply. Within a few drops of the equivalence point (about 50.29 mL), the pH goes up four to five pH units.
The pH at the equivalence point is determined by the concentration of the acetate ion. All of the acetic acid and sodium hydroxide have been reacted away. You can calculate the pH at the equivalence point easily. Just calculate the concentration of acetate ion (it's equal to the original moles of acetic acid we started with over the total volume of the solution - including both the acid and the base), then calculate the pH just like you would calculate the pH of a simple solution of sodium acetate.
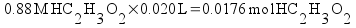
Since the moles of acetate at the equivalence point are equal to the moles of original acid, we can calculate the concentration.
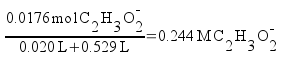
Now, find the pH of the acetate solution. Acetate ion is basic.
C2H3O2-(aq) + H2O(l) <--> HC2H3O2(aq) + OH-(aq)
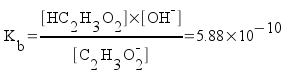
(Kb was found from the Ka of acetic acid, 1.7x10-5.)
Set up the equilibrium problem.
[C2H3O2-] = 0.244 - x
[OH-] = x
[HC2H3O2] = x
Plug in and solve.
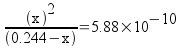
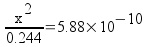
x = 1.20x10-5 = [OH-]
Use the water equilibrium to find pH.
14.00 = pH + pOH
pH = 14.00 - 4.92 = 9.08
The pH at the equivalence point, then, is 9.08. It's a basic pH because the acetate ion (which is the only thing present at the equivalence point that influences pH) is itself basic.
Phenolphthalein changes color over a range from pH 8 to pH 10. Since the equivalence point for the titration is pH 9.08, and since the pH changes extremely rapidly within a fraction of a drop around the equivalence point, the color change of phenolphthalein occurs immediately (clear to pink) as we reach the equivalence point. This is why phenolphthalein is a good indicator for this titration.
Beyond the equivalence point
There's not really much to say about the pH of the solution after the equivalence point has been passed. It's not very interesting, because the goal of the titration is to find the equivalence point. Once that's been found, it's a waste of materials to continue to titrate. (Unless you're titrating a polyprotic acid, in which case there will be multiple equivalence points!)
The pH in this region is controlled by the hydroxide ion from the sodium hydroxide, and can be calculated by finding out how much sodium hydroxide is left over after the reaction with the acetic acid. The contribution of the acetate ion to the pH in this region can largely be ignored thanks to the common-ion effect.
Another example: Titration of a weak base with a strong acid
We''ll look at another example of an acid-base titration - the titration of 10 mL of 0.25 M ammonia solution with 0.10 M hydrochloric acid solution. The titration reaction looks like this.
NH3(aq) + HCl(aq) <--> NH4Cl(aq)
You can easily calculate that the equivalence point of this titration occurs when 25 mL of hydrochloric acid have been added. A plot of the pH of this solution during the titration is shown below.
|
|
|
Illustration 2: pH of ammonia titration vs. amount of HCl solution added |
Like the other titration, this plot shows three distinct regions.
The buffer region for this titration occurs at a basic pH. The pH is controlled by the mixture of ammonia and ammonium ions (formed by the reaction of acid with ammonia) present. Again, these pH values may be calculated with the Henderson-Hasselbalch equation.
At the equivalence point, all ammonia has been converted to ammonium ion, and the pH of the solution can be calculated by finding the concentration of ammonium ion and using ammonium's ionization reaction in water. Ammonium ion is acidic, and the pH at the equivalence point is 5.20. (Try this calculation yourself!)
After the equivalence point, the pH is controlled by the excess hydrochloric acid present.
One thing to notice about this titration compared to the vinegar titration: Phenolphthalein will not work for this titration. It changes color over the range pH 8 to pH 10 - which means that phenolphthalein will turn clear before the equivalence point is reached! To monitor this titration, you'll need a different indicator.
Selecting an indicator
Using a pH meter for a titration can be tedious and time-consuming. If you don't want to always follow the progress of an acid-base titration using a pH meter, you'll need an indicator. The indicator you should use is one that changes color at or near the pH of the equivalence point of the titration. Usually you can find this out by calculating the pH at the equivalence point for a titration similar to the one you are going to perform. Also, you can do a test run with a pH meter and look for the steep change in pH that happens at the equivalence point and select an indicator that matches.
Summary
This note pack explained the behavior of weak acids and bases during titration. You should be able to describe the general features of these titration curves, and calculate the pH at the equivalence point for one of these titrations. You should also be able to determine the volume of acid or base required to reach the equivalence point. You should also be able to find an appropriate indicator for a titration based on the pH at the equivalence point.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.