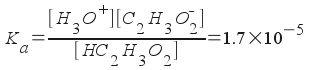
Introduction
We've discussed how to find the pH of solutions of strong acids/bases, solutions of weak acids/bases, and solutions of salts. Each time, we've dealt mainly with the case of only one compound present in solution. Now, we will look at solutions that contain multiple components. We will focus our attention on solutions that exhibit the so-called "common ion effect" - because they have certain desirable properties that make them important in both the biological and chemical fields.
The common-ion effect
The first thing we should do is define the common-ion effect . The common-ion effect occurs when several species in an equilibrium are all present initially. In a solution containing a weak acid, this equilibrium is important:
HA(aq) + H2O(l) <--> H3O+(aq) + A-(aq)
What if you prepared a solution that contained both the weak acid (HA) and its conjugate base (A-, usually present as a salt such as NaA)? If you made the assumption that the dissolved HA was at equilibrium before adding A-, then Le Chateleir's principle would tell us that the equilibrium would shift to the left (towards HA) as you added A-. If we put both HA and A- in at the same time, we'd logically conclude that the presence of A- would suppress the dissociation of HA.
Generalizing, the presence of the conjugate of an acid (or base) in solution suppresses the ionization of that acid (or base).
Showing the common-ion effect by calculation
We can easily demonstrate the common-ion effect with a simple calculation. Let's examine a solution prepared with both an acid and its conjugate base present.
What is the pH of a solution containing both 0.42 M acetic acid and 0.42 M sodium acetate? The Ka of acetic acid is 1.7x10-5.
This question can be answered by simply setting up the equilibrium and solving - just like we've done with numerous equilibrium problems in the past.
The equilibrium is (of course) the acetic acid / water equilibrium:
HC 2 H 3 O 2 (aq) + H 2 O( l ) <--> H 3 O + (aq) + C2H3O2-(aq)
We have an initial concentration of acetic acid present (0.42 M). We also have an initial concentration of sodium acetate present (0.42 M). We will ignore the sodium ion in sodium acetate because it does not influence our equilibrium. (Sodium ion is a spectator ion.) So, the initial concentration of acetate ion (which is the conjugate base of acetic acid) is 0.42 M.
Our next step in solving this problem is to write our equilibrium expression and express all concentrations in terms of one variable:
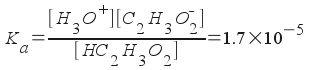
[H3O+] = x
[C2H3O2-] = 0.42 + x
[HC2H3O2] = 0.42 - x
We'll now make our usual assumption, since the acid dissociation constant is small compared to the initial concentration of the acid (so x is small). [Note: The assumption that x is small is even better in this case than it was when we calculated the pH of the 0.42 M acetic acid solution - since the presence of acetate will make the amount of acid that dissociates - x - even smaller!]
Plugging in and solving:
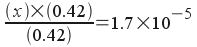
This equation is trivially easy to solve, and we get:
x = 1.7x10-5
Since x also equals the hydronium ion concentration (we defined it that way),
pH = -log(1.7x10 -5 ) = 4.77
Compare this to the pH of 0.42 M acetic acid, which was 2.57. Since the dissociation of acetic acid is what produces H3O+ in the first place, the less acid that dissociates, the lower the concentration of H3O+ - and the higher the pH! The pH of the solution that contains an equal amount of acetate ion and acetic acid is more than two pH units higher than the acetic acid solution. Therefore, we conclude (as expected), that the presence of acetate ion suppresses the ionization of acetic acid. This is the common-ion effect in action.
A "special" solution
The acetic acid / acetate solution we just investigated has several interesting (and important) properties. This solution is surprisingly resistant to pH changes. What would happen if you diluted the solution by adding water? Let's say we add enough water to make the solution 0.21 M in acetic acid and 0.21 M in acetate ion (half the initial concentration). If this were a solution of only acetic acid in water, we'd naturally expect the pH of the solution to go up, since we'd be diluting the acetic acid (and we'd be decreasing the concentration of hydronium ion, even though a slightly greater fraction of the acetic acid can dissociate in a more dilute solution). But what happens to the pH of this acetic acid / acetate solution?
If we set up and solve the equilibrium just as we did before with the 0.42 M / 0.42 M solution, we would get the following:
[H3O+] = x
[C2H3O2-] = 0.21 + x
[HC2H3O2] = 0.21 - x
Again, we assume x is small and solve:
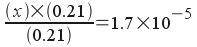
x = 1.7x10-5
pH = -log(1.7x10 -5 ) = 4.77
Notice that the pH of this solution didn't change when we diluted it. This isn't just a mathematical trick - you can verify this with a pH meter or indicator paper. [Note: you can eventually add enough water to "drown out" the contributions from the acid and base present. This would also mean that the assumption in our calculation above would be invalid!]
The solution of acetic acid and acetate ion has another interesting property - it resists pH change even when small amounts of acid or base are added. To illustrate this, let's calculate the pH of some of our solution after we've added a small amount of hydrochloric acid solution:
What is the pH of a solution made from 100 mL of 0.42 M acetic acid / 0.42 M sodium acetate mixed with 5.00 mL of a solution of 0.42 M hydrochloric acid?
We're again dealing with the acetic acid equilibrium:
HC 2 H 3 O 2 (aq) + H 2 O( l ) <--> H 3 O + (aq) + C2H3O2-(aq)
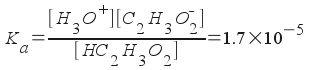
How does the hydrochloric acid affect the system? The hydrochloric acid (a strong acid) dissociates completely to form hydronium ion, which will effectively combine immediately with acetate ion (a weak base) to form more acetic acid. [Note: Remember that acetic acid is a weak acid, and exists mostly in water as molecules]
So, we need to figure our the new concentrations of acetic acid and acetate ion after the addition of hydrochloric acid. To do this, we first need the amount of each species present. In other words, we must find out how many moles of each species there are.
Acetic acid: (0.42 mol/L) × (0.100L) = 0.042 mol HC2H3O2
Acetate ion: (0.42 mol/L) × (0.100L) = 0.042 mol C2H3O2-
Hydrochloric acid: (0.42 mol/L) × (0.00500L) = 0.0021 mol HCl
Hydrochloric acid will react with the base C2H3O2- to form acetic acid, so we account for that:
Acetic acid: 0.042 mol + 0.0021 mol = 0.0441 mol HC2H3O2
Acetate ion: 0.042 mol - 0.0021 mol = 0.0399 mol C2H3O2-
Hydrochloric acid: Reacted away.
Since the equilibrium constant expression requires molar concentration, we now find the initial molar concentration of each species (after accounting for hydrochloric acid's reaction). Remember that there are 105 mL of total solution!
Acetic acid: [HC2H3O2] = 0.0441 mol / 0.105 L = 0.42 M
Acetate ion: [C2H3O2-] = 0.399 mol / 0.105 L = 0.38 M
Now, we can solve the equilibrium by writing equilibrium concentrations of each species involved in the equilibrium in terms of one variable and solve:
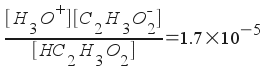
[H3O+] = x
[C2H3O2-] = 0.38 + x
[HC2H3O2] = 0.42 - x
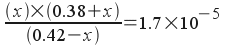
Since the initial concentrations of acetic acid and acetate are much greater than the acid dissociation constant, we can assume that the amount of acid that dissociates (x) is small and simplify the equation:
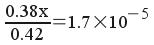
Solving:
x = 1.879x10-5 M = [H3O+]
pH = 4.72
Compare this to the pH of the original acetic acid/sodium acetate solution, 4.77. Adding the hydrochloric acid to this solution only changed the pH by 0.05 pH units! If you'd added the 5 mL of 0.42 M hydrochloric acid to distilled water, the pH would be 1.70. [Note: See if you can verify this on your own - it's a simple exercise.]
So, this solution of acetic acid and sodium acetate possesses some remarkable pH properties. We call solutions that resist pH change from dilution or from the addition of acid or base buffers , and acetic acid / acetate is but one example of this type of solution.
Buffer solutions
A buffer solution can be formed one of two ways:
Making a solution containing a weak acid and its conjugate base (e.g. acetic acid and sodium acetate).
Making a solution containing a weak base and its conjugate acid (e.g. ammonia and ammonium chloride) .
These solutions, while differing in their actual pH values, will all tend to:
Resist pH change when diluted.
Resist pH change when small amounts of strong acid or base are added.
Since many chemical reactions - especially biochemical ones - will only proceed at certain concentrations of hydronium ion (in other words, certain pH values), buffers are vitally important both in biology and the chemical industry. You deal with buffers constantly without even thinking about it. Your blood is a (somewhat complex) buffer solution. Some drugs are delivered in "buffered solutions". Water in a lake or pond typically contains enough weak acid/base and salts to be buffered (else, one dose of acid rain would kill everything in the pond!). You've used pH buffers in the lab to calibrate the pH meter. Buffers are everywhere.
Since buffers are so important, there's been much research done and many books published on buffers. We'll summarize some of the most important findings.
Buffer solutions tend to buffer best (hold their pH best) within a range of +/-1 pH unit from the pKa of the acidic species in the buffer. For example, acetic acid / sodium acetate buffers best at pH values near 4.77 - the pKa of acetic acid. Ammonia / ammonium nitrate buffers best at pH values near 9.25 - the pKa of ammonium ion.
So, if you want to select a buffer system for a desired pH, you look at the buffer system's pKa value. Sodium acetate / acetic acid would be a good buffer for pH 5, but a poor choice for pH 10. On the other hand, ammonia / ammonium nitrate would be a good choice for pH 10, but a poor choice for pH 5.
Since buffers are so commonplace, there's a simple equation for calculating their pH. In fact, it's a solution of the equilibrium problem - only in a general form. This solution is called the Henderson-Hasselbalch equation, and can be used for buffers. For a buffer system,
|
HA(aq) |
+ |
H2O(l) |
<--> |
A-(aq) |
+ |
H3O+(aq) |
|
acidic species |
|
|
|
basic species |
|
|
... the pH can be found with:
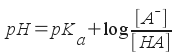
The pKa is the pKa of the acidic species in the buffer. The molar concentrations in the formula are equilibrium constants, but weak acids and bases don't dissociate much in water, so you can plug the initial concentrations in without adding much error. This is equivalent to assuming that x is small (as we've been doing in most of our weak acid/base calculations).
We can write this a little more generally if we like:
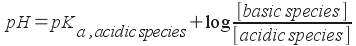
This form covers any buffer. To see how this equation simplifies calculation , let's look at our last example (the acetic acid / acetate plus hydrochloric acid). I'll omit the preliminaries and start off with the initial concentrations of acetic acid and acetate in the mixture:
Find the pH of a buffer solution containing 0.42 M acetic acid and 0.38 M sodium acetate.
This is a buffer, so we use the Henderson-Hasselbalch equation. The acidic species is (of course) acetic acid, while the basic species is acetate ion.
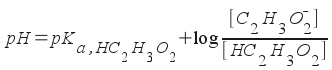
Since the K a of acetic acid is 1.7x10 -5 , the pK a of acetic acid is 4.77 . Now, we simply plug in and solve!
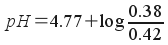
pH = 4.77 + (-0.0434)
pH = 4.73
This is approximately the same as we found the last time - the difference is due to rounding). Note that calculating the pH of a buffer solution using the Henderson-Hasselbalch equation is quite easy!
Summary
We've discussed the common-ion effect and certain solutions that exhibit the common-ion effect - namely, buffers. You should understand that the common-ion effect works to suppress the ionization of weak acids and bases. You should be able to qualitatively and quantitatively determine the effect of the presence of a common ion on pH. You should know that certain solutions exhibiting the common-ion effect are buffers - solutions that resist pH change. You should be familiar with the properties of buffers, how to choose a buffer, and how to use the Henderson-Hasselbalch equation to calculate the pH of a buffer.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.