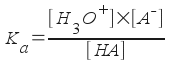
Introduction
We've discussed acids and bases on and off throughout your general chemistry courses. We first learned that acids increase the concentration of hydronium ion in water, and bases increase the concentration of hydroxide ions. You also learned that strong acids and bases completely ionize in solution while weak acids and bases do not. What we didn't discuss at the time was this - to what extent do weak acids and bases dissociate and how do we know how weak one acid or base is compared to another. Now that we understand equilibrium, we are ready to discuss the behavior of weak acids and bases in more detail. We will even be able to extend the discussion to things you traditionally don't think of as having acidic and basic properties. We will also discuss buffer solutions, which are solutions with special pH properties that are vitally important in biological systems.
The equilibrium of a weak acid
Why don't weak acids dissociate completely? The simple answer is that they come to equilibrium instead. You can write the equilibrium process for a weak acid dissolved in water this way:
|
HA(aq) |
+ |
H2O(l) |
<--> |
H3O+(aq) |
+ |
A-(aq) |
|
weak acid |
|
|
|
|
|
conjugate base |
HA is our generic weak acid, and A- is its conjugate base. (What acid/base definition are we using here?). We can easily write an equilibrium expression for the above process.
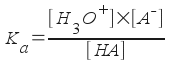
[Note: Water is the solvent in this reaction, so it does not appear in the equilibrium expression. The concentration of water is effectively constant in a dilute aqueous solution.]
The equilibrium constant Ka is called the acid dissociation constant. The value of this constant relates directly to the strength of the acid. A very weak acid (one that does not dissociate much at all in water to form H3O+) will have a very small Ka. Hypobromous acid, HBrO, is a rather weak acid. The Ka for hypobromous acid is 2.06x10-9. [Write the equilibrium equation for the dissociation of hypobromous acid in water.] The stronger the acid, the larger the Ka.
You may also find a "pKa" value in some reference books instead of a Ka value. The pKa is simply the negative logarithm of the Ka value. The pKa of hypobromous acid is -log( 2.06x10-9 ), or 8.69.
What about strong acids? You can write an equilibrium expression for strong acids that looks just like the above. Strong acids, though, have an extremely large - effectively infinite - value of Ka. This should make perfect sense to you, as a very large equilibrium constant would mean that the equilibrium was dominated by products. In other words, the equilibrium is dominated by hydronium ion and the conjugate base A-.
The equilibrium of a weak base
We can treat weak bases similarly to the weak acids we discussed above. The weak base also comes to equilibrium. You can write the equilibrium process for a weak base dissolved in water this way:
|
B(aq) |
+ |
H2O(l) |
<--> |
BH+(aq) |
+ |
OH-(aq) |
|
weak base |
|
|
|
conjugate acid |
|
|
B is our generic weak base, and BH+ is its conjugate acid. (We're using the same acid/base definition that we used for our weak acid). We can easily write an equilibrium expression for the above process.
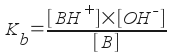
[Note: As before, water is the solvent in this reaction, so it does not appear in the equilibrium expression. The concentration of water is effectively constant in a dilute aqueous solution.]
The equilibrium constant Kb is called the base dissociation constant. As for acids, the value of this constant relates directly to the strength of the base. A weak base (one that does not dissociate much in water to form OH-) will have a small Kb. Methylamine, CH3NH2, is considered a weak base. Its Kb is 3.70x10-4. [Write the equilibrium equation for the dissociation of methylamine in water.] The stronger the base, the larger the Kb.
What about strong bases? As for strong acids, you can write an equilibrium expression for strong bases that looks like the above. Strong bases have a large - effectively infinite - value of Kb. This means that the equilibrium is dominated by hydroxide ions and the conjugate acid BH+.
You may find that if you go to look up the Kb for a base, you might sometimes find a Ka or a pKa! What you're looking at is the Ka (or pKa) for the conjugate acid of your base. You can find the K b easily enough , using a simple relationship between Ka, Kb, and the water equilibrium constant Kw. In an aqueous solution, these three are related by:
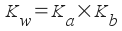
... where Ka and Kb are the acid and base dissociation constants of a conjugate pair.
The Ka of the conjugate acid of methylamine is:
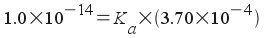
Ka, conjugate acid of methylamine = 2.70x10-11
pKa, conjugate acid of methylamine = 10.57
You might find either of the above values in a reference book. See if you can find the value of Kb given the values above.
Sample pH calculations for weak acids and bases in water
Since weak acids and bases come to equilibrium with their conjugates in water, the calculation of the pH of a weak acid or base simply requires you to solve an equilibrium problem. Let's look at the case of a common weak acid dissolved in water.
What is the pH of an 0.42 M aqueous solution of acetic acid, HC2H3O2? Ka for acetic acid is 1.7x10-5.
To solve this problem, we must first write out the equilibrium between acetic acid and water - the dissociation reaction. We must also write our the equilibrium expression.
|
HC2H3O2(aq) |
+ |
H2O(l) |
+ |
C2H3O2-(aq) |
+ |
H3O+(aq) |
|
weak acid |
|
|
|
conjugate base |
|
|
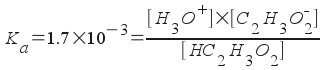
The problem asks us to calculate pH, which relates to [H3O+]. So, we must find [H3O+]! This is a simple equilibrium problem like we've done before. Assign [H3O+] = x and write all equilibrium concentrations in terms of one variable.
[H3O+] = x
[C2H3O2-] = x
[HC2H3O2] = 0.42 - x
When we plug into the equilibrium expression, we get:
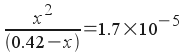
You should recognize this as a quadratic equation. However , there's a shortcut we can take in our solution if we use our heads and look carefully at the value of the equilibrium constant. How much of the acetic acid actually dissociates? The equilibrium constant is 1.7x10 -5 , we can say that, relative to 0.42 M , very little acetic acid dissociates . If we make that assumption, then we can say:
0.42 - x = 0.42
[The concentration of acetic acid doesn't change much. The vast majority of the acid remains as undissociated molecular HC2H3O2.]
Now, we must solve a much simpler equation:
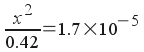
Solving,
x2 = 7.14x10-6
x = 2.672x10-3 M = [H3O+]
The pH, then, is -log(2.672x10-3) = 2.57
Let's do some sanity checks on our answers. Do they make sense?
2.672x10-3 (the value of x) is indeed much smaller than 0.42.
The pH of this solution of acetic acid is less than 7 - it's acidic.
Our answers, then, appear to make sense.
Note: If you'd solved the quadratic equation with the quadratic formula, you would have obtained x = 2.664x10-3, and the pH would have still equaled 2.57.
Let's try a base .
What is the pH of a 0.42 M aqueous solution of ammonia, NH3? The Kb of ammonia is 1.8x10-5.
[Try this one on your own before looking at the solution below!]
As for the acid case, write out the equilibrium between ammonia and water, then write our the equilibrium expression.
|
NH3(aq) |
+ |
H2O(l) |
+ |
NH4+(aq) |
+ |
OH-(aq) |
|
weak base |
|
|
|
conjugate acid |
|
|
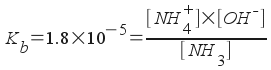
The problem asks us to calculate pH, which relates to [H3O+]. H3O+ does not directly appear in the equilibrium, but it is related to [OH-]. So, we must find [OH-]! Assign [OH-] = x and write all equilibrium concentrations in terms of one variable.
[NH4+] = x
[OH-] = x
[NH3] = 0.42 - x
When we plug into the equilibrium expression, we get:
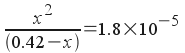
As before, this is a quadratic. The equilibrium constant, though, is 1.8x10-5. We can say that, relative to 0.42 M, very little ammonia dissociates . If we make that assumption, then we can say:
0.42 - x = 0.42
[The vast majority of the base remains as undissociated molecular NH3.]
Now, we solve:
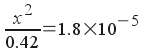
Solving,
x2 = 7.56x10-6
x = 2.7495x10-3 M = [OH-]
We need to relate this to the concentration of hydronium ion, [H3O+]. Since this ammonia is in aqueous solution, we use the water equilibrium like we did when finding the pH of a solution of strong base.
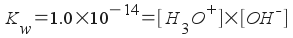
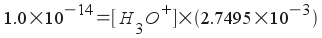
[H3O+] = 3.637x10-12 M
The pH, then, is -log(3.637x10-12) =11.44
Again, let's do some sanity checks on our answers. Do they make sense?
2.7495x10-3 (the value of x) is indeed much smaller than 0.42.
The pH of this solution of ammonia is greater than 7 - it's basic.
Our answers, then, appear to make sense.
Summary
In this note pack, we've discussed the equilibrium of simple solutions of weak acids or bases dissolved in water. We've defined the dissociation constants, Ka and Kb, and discussed how you might find values of these in reference books. You should be able to, using the Bronsted-Lowry definition, write the reaction of a weak acid or base with water, write our the expression for the equilibrium constant, and use them to solve for the pH or a weak acid or base solution. You should also realize that you can often simplify your equilibrium calculations for these solutions if the dissociation constant is much smaller than the initial concentration of the weak acid or base.
All original site content ©2007 Charles Taylor. Page updated: December 12, 2007.