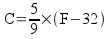
Introduction
Units are critical in all measurements. If you don't tell someone a unit when you tell them a number, they're not likely to know what you're talking about. This note pack will discuss units as they're used in the sciences - with emphasis on the units you'll be responsible for in this chemistry course.
American units vs. the metric system
In America, we're used to using so-called "English" or "US customary" units. This is unfortunate because these units are irregular - and thus harder to use. Scientists and most of the rest of the developed world use the metric system (the "SI" system). The main advantage of SI units is that they're constructed in such a way that most units can be derived from simple base units. The SI base units we need for chemistry class are shown below.
|
Quantity |
SI base unit |
Symbol |
|---|---|---|
|
Amount of substance |
mole |
mol |
|
Length |
meter |
m |
|
Mass |
kilogram |
kg |
|
Temperature |
Kelvin |
K |
|
Time |
second |
s |
Your textbook lists a few others, but the ones above are your primary concern for general chemistry. These base units are not the only units you'll use, but other units can be defined in terms of these base units.
The kilogram is a bit of an oddity. Even though it is considered the base unit of mass in the SI system, we treat the gram as if it is the base.
Temperature: Celsius, Fahrenheit, and Kelvin
The three temperature scales you've probably encountered are the Fahrenheit scale, the Celsius scale, and the Kelvin scale. In the US, we use the Fahrenheit scale most of the time. In science, we use the Celsius and Kelvin scales regularly and almost never use the Fahrenheit scale.
|
oF |
oC |
K |
Notes |
|---|---|---|---|
|
-459.67 |
-273.15 |
0 |
Absolute zero - as cold as it gets! |
|
-320.44 |
-195.8 |
77.35 |
Boiling point of liquid nitrogen |
|
-108.4 |
-78.00 |
195.1 |
Sublimation point of dry ice |
|
-40 |
-40 |
233 |
An interesting temperature ... |
|
0 |
-18 |
255 |
Zero Fahrenheit |
|
32 |
0 |
273 |
Freezing point of water |
|
77 |
25 |
298 |
Room temperature |
|
86 |
30 |
303 |
A warm day |
|
98.6 |
37.0 |
310.2 |
Normal body temperature |
|
212 |
100 |
373 |
Boiling point of water |
|
621.43 |
327.46 |
600.61 |
Melting point of lead metal |
|
1474 |
801.1 |
1074 |
Melting point of sodium chloride |
These reference points should give you something of a feel for how the Celsius and Kelvin scales compare to the Fahrenheit scale. Here's how to convert back and forth between the units.
Fahrenheit to Celsius:
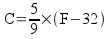
Celsius to Fahrenheit:
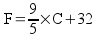
Celsius to Kelvin:
K = C + 273.15
All numbers in these conversion equations are exact !
To get from Celsius to Kelvin you simply add a number. This means that a Celsius degree is the same size as a Kelvin degree. So why the two scales? The Kelvin scale is an absolute scale, while Celsius and Fahrenheit are relative scales. Zero on the Kelvin scale is as cold as it can get (you'll learn more about how this temperature was discovered when we discuss gases). The Kelvin scale is defined so that there aren't any negative Kelvin temperatures.
The metric prefixes
SI units can be scaled to meet your needs. If you're trying to describe the length of your finger, the meter is probably too big. (Your finger might not be but 0.08 meters long!). Instead, you'd prefer to use a unit that would give you a nice round number - say, 8 centimeters. You can scale a base unit to whatever magnitude you like using one of the metric prefixes, like centi-. Here are some common metric prefixes you will need to know.
|
Prefix |
Symbol |
Multiplier |
|---|---|---|
|
mega- |
M |
106 |
|
kilo- |
k |
103 |
|
deci- |
d |
10-1 |
|
centi- |
c |
10-2 |
|
milli- |
m |
10-3 |
|
micro- |
|
10-6 |
|
nano- |
n |
10-9 |
|
pico- |
p |
10-12 |
All of these multipliers are exact numbers.
These aren't all of the metric prefixes, but they're the ones you're likely to run into in science courses. Most of the common prefixes are used to make smaller units than the base unit. We tend to deal with small amounts of chemicals in our labs.
So how do the prefixes work? Centi-, for example, means 10-2. So a centimeter is 10-2 (or 1/100) meters. Flipping it around, it takes 102 centimeters (100 centimeters) to make a meter. Kilo- means 103. A kilometer is 103 meters, or 1000 meters.
Dimensional analysis and unit conversions
So how do you convert between one unit and another? You might have been taught some shortcuts back in grade school for converting back and forth between centimeters and kilometers and so forth, but the method we will discuss now is general . It will work for all cases - including those where grade-school shortcuts will fail.
The method is called dimensional analysis. Dimensional analysis is a process of converting a quantity from one unit to another by using conversion factors . Conversion factors are equalities (or ratios) that relate one unit to another. For example, we said that the prefix centi- meant 10-2, and a centimeter was 10-2 meters. We also said that the prefix kilo- meant 103 , and a kilometer was 103 meters . We can express these relationships as conversion factors.
1 cm = 10-2 m
1 km = 103 m
These two expressions are conversion factors to convert from one unit to another. We can also express these conversion factors as ratios:
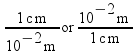
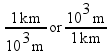
Since the numerator and denominator in these ratios are equal to each other, all of these fractions are mathematically equal to 1. Multiplying by one of these factors will change the units of a measurement, but not the measurement itself.
We can use these ratios to convert from one unit to another. For example, let's convert a measurement of 1.205 m to cm .
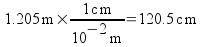
The meter units cancel out and we're left with centimeters - which is what we want. We used a conversion factor that canceled out the unwanted unit and left us with the desired one. Since multiplying by the conversion factor is mathematically equivalent to multiplying by 1, we have not changed the actual value of the measurement. All we have done is to change the unit.
Now let's look at a trickier example. Convert a measurement of 120.5 cm to km.
You may worry that you don't have a conversion factor that goes directly from centimeters to kilometers. You could derive one from the table of multipliers earlier in this note pack, but there's a simpler way to deal with the problem. Convert to the base unit (the unit with no prefix) first . Then, convert from the base unit to the desired unit .
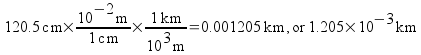
This time we cancel out centimeters to convert to meters, then we multiply by another conversion factor to convert to our desired unit, kilometers. All units cancel out except for our desired unit. The most important thing to remember in dimensional analysis is to check and make sure your units cancel our properly. This means that you should write down units with every number in your calculations.
It's important that you learn to use dimensional analysis right now. We will use dimensional analysis for much more than converting from one metric unit to another. Conversion factors can be used to convert from the amount of a chemical before a reaction to the amount of a different chemical after a reaction. This is called stoichiometry, and we will discuss it later in the course.
Practice using dimensional analysis to do unit conversions - especially if you've been using shortcut methods.
Derived units - volume and density
Units can be derived from base SI units by simply adding prefixes. Units can also be derived from base SI units by combining multiple SI units. In chemistry, two of these derived units are particularly important : volume and density.
The volume of an object is simply how much three-dimensional space an object occupies. In geometry class (a long time ago), you probably learned that the volume of a box is equal to its length times its width times its height.
|
|
|
|
Illustration 1 - Volume of a box |
|
If you measure the sides of a box with SI base units (meters), you'll find that the area has a unit of cubic meters, or m3. The cubic meter is the SI unit of volume, and it the volume of a cube one meter on each side. This is a large volume compared with what we usually deal with in the chemistry lab, so we use more convenient units based on SI units. We use the liter (L) and milliliter (mL) in chemistry lab.
|
1 L = 1000 mL = 1 dm3 1 mL = 1 cm3 |
You should remember at least one of these conversion factors. The second is likely the most useful one, as we will normally deal with liquid volumes measured in milliliters.
The other important derived unit we will discuss is the density. Density is a term you're probably familiar with. We say that small but heavy objects are dense. The density of an object is its mass per unit volume. In base SI units, density would be expressed as kg/m3 (kilograms per meter cubed - the SI unit of mass per SI unit of volume). As with volume, though, we're used to working with smaller units in the laboratory. In most cases, you'll deal with density as grams per milliliter, or g/mL.
Pure water has a density (at 4 oC) of 1.000 g/mL. This is a reference point you should remember, as you can use it to relate to other substances.
Summary
In this note pack, we've discussed some of the units that we will use in our study of chemistry. You should be familiar with these units - which means that you should know the SI base units, the prefixes, and the common derived units. Unfortunately, this requires some memorization, but soon the units will become second nature to you and you won't have to worry about what unit you should use for a measurement or how to get from one unit to another. You've also been introduced here to the concept of dimensional analysis. Dimensional analysis is an extremely powerful tool useful for doing a majority of the calculations you're responsible for in college chemistry. It's also applicable to the world outside of chemistry.
All original site content ©2007 Charles Taylor. Page updated: November 28, 2007.