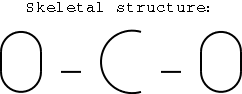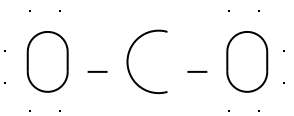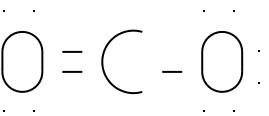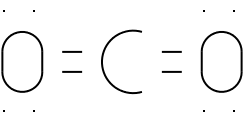Simple Lewis Structures
Introduction
In the previous note pack, you learned
some about Lewis dot structures, which represent chemical compounds
by showing how electrons are distributed between the molecules. We
will now learn how to draw Lewis dot structures ("Lewis
structures", or "Lewis formulas") for most molecules
and ions. The reason we do this is that the Lewis structure gives us
key information about the molecule. Given a set of molecules, if
we're able to draw the Lewis structure we can tell which molecule
should have the higher boiling point, what each molecule might
dissolve in, etc. And we get all of this without having to
handle the chemical in the lab!
The basics
In a previous note pack, we discussed
the basics of drawing Lewis structures. Dots represent s and
p valence electrons. A single line represents a pair of
electrons shared between two atoms, while double and triple lines
represent two and three pairs of electrons shared - single, double,
and triple bonds . Now we
will move on to talk about how to draw Lewis structures for complex
species - those with more than two atoms.
Lewis
structures - step by step
You
can use essentially the same set of steps to draw the Lewis structure
for almost anything. We'll modify these as we learn a few
more things about the structure of atoms, but this discussion will be
a good starting point when you need a structure. We will illustrate
the process with a simple example - carbon dioxide (CO2).
1) Count the valence electrons.
You can do this by writing the Lewis dot formula for each atom in
the compound, or you can use the periodic table to get the number of
valence electrons.
|
|
You don't have to draw the Lewis structures - but
if you're new to this, it's good practice!
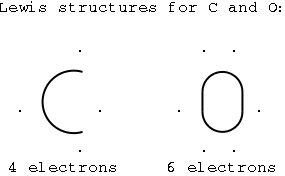
Carbon is in group IVA, which has 4 valence electrons.
Four are unpaired, and carbon is likely to form four bonds.
Oxygen is in group VIA, which has 6 valence electrons.
Two are unpaired, and oxygen is likely to form two bonds.
|
|
Illustration 1 - Lewis structures of C and O
|
|
If
the species is an ion ,
you need to add electrons (if the ion is negatively charged) or
subtract electrons (if the ion is positively charged). Our example
molecule has a total of 16 valence electrons, four from
the carbon, and twelve from the two oxygens. This molecule is
neutral (uncharged), so we don't need to add or subtract any
electrons.
2) Write a skeletal structure.
You can do this by connecting atoms with a single bond. So how do you
arrange the atoms? Sometimes you will be told. Sometimes the
central atom is obvious - in SF6, sulfur is the central
atom. Complicated molecules may have more than one "center".
Unless you're told otherwise, you can draw a structure by arranging
the atoms so that the least electronegative atom is in the center
of the structure.
|
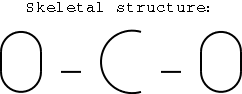
|
The electronegativity of carbon (2.5) is less than that of
oxygen (3.5), so put carbon in the center.
Since there's only one carbon and two oxygens, it also
seems logical to put carbon in the center.
Each atom connects to the next with a single bond.
|
|
Illustration 2 - Skeletal structure of CO2
|
|
In our example, carbon is the central
atom and we've bonded it using single bonds to each oxygen.
3) Distribute the electrons .
First, give electrons to the outer atoms. Then, distribute the
remaining electrons (if any) to the inner atoms until you run out.
Remember that each single bond in your skeletal structure contains
two electrons , so
subtract those out before distributing.
|
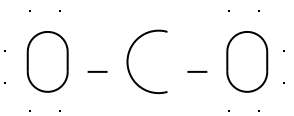
|
We've made two single bonds - this uses four electrons.
After we fill the valence shells of each of the oxygen
atoms, we've used all 16 valence electrons.
|
|
Illustration 3 - Electrons distributed around CO2
|
|
We've used all
sixteen electrons, so we now have to evaluate whether this is an
acceptable structure for carbon dioxide.
4) Check to see
if each atom in the structure has eight valence electrons - if not,
rearrange electrons. The main reason an atom bonds with another
is to get a stable electron configuration - in other words, they want
filled valence shells like the noble gases have. We're only going to
concern ourselves with the 's' and 'p' subshells (which can contain a
total of eight electrons - two in the 's' subshell and six in the 'p'
subshell) when drawing Lewis dot formulas. So, we need to check our
structure to make sure that each of our atoms sees eight
electrons. We call this the octet rule and we check to
make sure each atom in our structure has an octet - eight
valence electrons.
There
is a common exception to every atom getting eight valence electrons -
hydrogen. Since hydrogen has only an n=1 shell, that shell is full
once hydrogen's 's' orbital is full. That means the hydrogen
needs only two electrons total. When hydrogen forms a
molecule, it shares its one electron with another atom, forming only
one bond.
Let's count up the
electrons that each atom sees in our structure in Illustration 3.
Each single bond contains two electrons seen by each atom
involved in the bond.
|
Atom
|
Electrons from bonds
|
Other electrons
|
Total electrons
|
|
Carbon
|
4 (two single bonds)
|
0
|
4
|
|
Oxygen (left)
|
2 (one single bond)
|
6
|
8
|
|
Oxygen (right)
|
2 (one single bond)
|
6
|
8
|
We can see a
problem immediately with our structure - carbon sees only four
electrons, so it doesn't have an incentive to bond. We need to
rearrange the electrons so that each atom has an octet.
How do we do this? We need to share more electrons - by creating
more bonds.
We will add a
double bond to the structure and allow oxygen and carbon to share
more electrons.
|
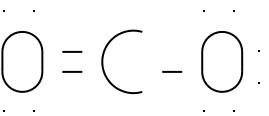
|
We've added a double bond between a carbon on the left and
an oxygen.
The carbon on the left has only four other electrons
around it - this gives it eight.
Again, we've run out of electrons before getting to
carbon.
|
|
Illustration 4 - Proposed structure for CO2
|
|
Let's check this
structure just like the last one.
|
Atom
|
Electrons from bonds
|
Other electrons
|
Total electrons
|
|
Carbon
|
6 (one single bond, one double bond)
|
0
|
6
|
|
Oxygen (left)
|
4 (one double bond)
|
4
|
8
|
|
Oxygen (right)
|
2 (one single bond)
|
6
|
8
|
Carbon still has only six electrons,
but it should now be obvious what to do to give carbon eight - bond
the other oxygen with a double bond. We could use a triple
bond to the first oxygen here, but since there's no reason to suppose
one oxygen atom bonds differently from another in this situation, we
give the other oxygen a double bond. Using a second double bond
gives us the following structure.
|
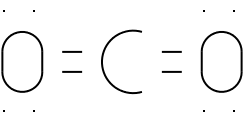
|
|
|
Illustration 5 - Lewis structure of CO2
|
|
Let's check this structure.
|
Atom
|
Electrons from bonds
|
Other electrons
|
Total electrons
|
|
Carbon
|
8 (two double bonds)
|
0
|
8
|
|
Oxygen (left)
|
4 (one double bond)
|
4
|
8
|
|
Oxygen (right)
|
4 (one double bond)
|
4
|
8
|
Carbon and both oxygen atoms have an
octet. This structure is likely to be the correct one.
Practice
Practice writing Lewis structures for
the following simple species. Use the four steps we've outlined in
this note pack. Try to do each without looking in the book for the
answer - use only the periodic table.
|
CO
|
N2
|
NH3
|
NH4+
|
OF2
|
CH2O
|
CH2F2
|
Summary
We've discussed a simple method to
write and draw Lewis structures of simple molecules and polyatomic
ions. You should now be able to draw simple structures easily and to
be able to make an attempt at drawing structures for more complex
molecules.
All original site content ©2007 Charles Taylor. Page updated: November 28, 2007.