Scientific Method r 7
Introduction
One of the first
things to do when you approach a new field is to get familiar with
the terminology people in the field use. As you start this chemistry
course, you need to learn m to use the terms that chemists use.
Since chemistry is a science, that means you need to learn to talk
and think like a scientist.
If this is not
your first science course, much of the material in this note pack
will be a review. Even so, read through it anyway. Science is a
group effort, and it's vitally important that we use the same words
to describe the same things when we're doing science.
Science and the scientific method
You can simply
define science itself as the systematic study of the natural world.
But that definition is missing something. The way that
knowledge is obtained is a vital part of science. Scientists use
something called the scientific method to gain knowledge.
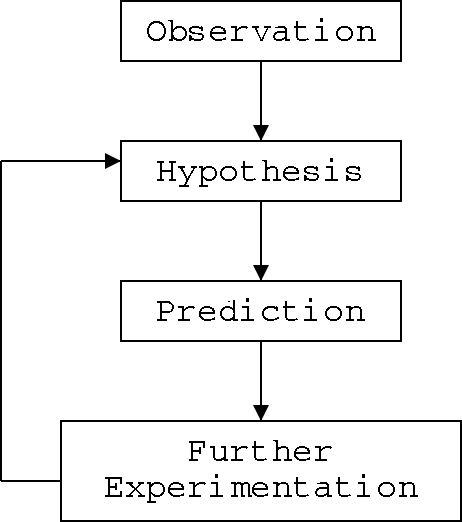
The scientific method is shown on the
flowchart in Illustration 1. We start with observation or
information-gathering. We observe and document something that is
happening. Once we've gathered enough information, we try to explain
what is happening - we create what scientists call a hypothesis.
A hypothesis is an explanation
of the thing we are observing. It tells us why (we think) the thing
is happening, and gives us a direction for future research.
After we develop a hypothesis, we don't
just sit back and congratulate ourselves on a job well done. Good
hypotheses make predictions that can be tested. (Many
scientists will tell you that if there's no way to test something,
then that something isn't science!) So we do further experiments -
giving ourselves more observations to compare to our hypothesis.
We also deliberately try to falsify
our hypothesis - by setting up tests where we can evaluate whether
the hypothesis accurately explains what we think it does.
After that, we tell other scientists
about our hypothesis, so they can try to come up with ways to
break it. It sounds stressful (and can be), but this is the only way
we can ensure that we're not fooling ourselves.
So what if there's a problem with our
hypothesis - something that doesn't fit into our explanation?
Depending on how big the problem is, we can either modify our
hypothesis or scrap it completely for a new one. Most hypotheses
are changed at least a little after they're developed, and there's no
shame in admitting that we have had to change our hypothesis based on
new evidence.
|
|
The scientific method starts with observations of the real
world and attempts to explain them. These observations should be
reproducible, since humans can make measurement errors.
The explanation (the hypothesis) is then tested based on
the predictions about similar phenomena that the explanation
predicts.
If the explanation doesn't predict the results of the new
experiments, the explanation must be modified or (if it's too far
off the mark) rejected.
Don't think of the scientific method as a list of
things to do. Think of it as an ongoing process!
|
|
Illustration 1: The scientific method
|
|
Terminology: hypotheses, theories,
and laws
In everyday language, a "theory"
is the name given to a conjecture or a guess. A "law", in
everyday language, is something that's always true, and nobody
outside of science routinely uses the word "hypothesis".
Unfortunately, science uses all three
of these terms, and the meaning of these terms to scientists is very
different from the common meaning. This leads to quite a bit of
confusion when people with no scientific training at all look into
science without realizing the terminology differences.
In science, the word that's closest to
"conjecture" or "guess" is hypothesis. A
hypothesis is an explanation of something that's so far supported by
only a small set of data. It's quite common for a hypothesis to
either need revision or be scrapped entirely for a better idea.
The theory, on the other hand,
is also an explanation of something. However, it is an
explanation that has been tested, revised, and retested until most
scientists regard it as the best explanation available for the
something in question. That's a point worth restating; a
scientific theory is the best currently available explanation of
data. That's not so say that scientific theories are never
changed (all explanations in science must agree with new data - else
the explanations need revision), but that they don't get changed on a
whim. (To put it another way: if you are able to gather good data
that overturns a well-established scientific theory, you will earn
yourself a place in the history books.)
That leaves us with law. Unlike
bills in Congress, theories do not get promoted to laws when they're
signed by the President of Science! Laws in science are
simply descriptions of some regularity in nature. Often these
are given as equations, such as the well-known law of gravity.
Laws are also not necessarily always
true; they often have well-defined limitations. In chemistry, a
simple equation called the ideal gas law
describes how the volume of a gas relates to the pressure and
temperature. But the ideal gas law is limited to conditions well
away from the pressure and temperature at which the gas would change
to the liquid state. The ideal gas law fails to give correct volumes
at these conditions.
A key difference
between laws and theories is that laws do not explain
anything. They merely describe a phenomenon. Explanations
are left to theories. The ideal gas law does not explain why the
volume of a gas depends on pressure and temperature. The explanation
of gas behavior is contained in kinetic theory, which (along
with the ideal gas law) will be discussed later in this course.
An example of the scientific method:
combustion
Let's say you were a scientist back in
the old days who was interested in combustion. When you burn a
material like wood, you get ash. If you look at the ash, it appears
smaller than the original wood. If you weigh the ash, you notice
that the ash is much lighter than the original wood. You try
to burn some other substances - some burn and some don't. Of the
ones that burned (leaves, paper, hair, meat, etc.), you notice that,
like wood, the remaining ash appears smaller and weighs less than the
original material.
From these observations, you'd probably
conclude that the process of burning releases something from the
material that was burned. Materials that burn (combustibles) must
contain this substance, while noncombustibles don't. The lost weight
would be evidence of the substance's presence in the original
material.
This explanation is a scientific
hypothesis - an explanation of some of the features of combustion.
One prediction that comes from this hypothesis is that the ash of a
burned material should always weigh less than the unburned material.
So, you conduct more experiments, and
eventually start burning some of the more easily combustible metals
like calcium or magnesium. Although the ash from these metals
appears similar to the ash from the earlier materials, you
find that it weighs more than the original metal does - every
time.
Going back and looking at your earlier
experiments, collecting both the ash and the gases released by
the burning wood shows you that the metals aren't really an anomaly.
The combined weight of the gases and the ash is actually larger than
the original weight of the wood. You find the same to be true for
the other substances you had burned.
You notice that the data now points in
a different direction from your original hypothesis. Combustion must
be the combination of a substance with the combustible
material, not the loss of a substance already contained in the
material. Using this new hypothesis, you might set out to identify
what this material was, and you're off to do more science!
This little example is a simplified
version of what did happen around 1800, when the phlogiston
theory (which held that combustion was caused by the loss of a
substance called "phlogiston" from a combustible) was
replaced by the modern theory of combustion - that burning was the
combination of a substance with oxygen gas.
Summary
This note pack gives you a basic
introduction to the scientific method and the terms used by
scientists to describe their work. You should be familiar with the
steps of the scientific method and the terms hypothesis, theory, and
law.
All original site content ©2007 Charles Taylor. Page updated: November 28, 2007.
