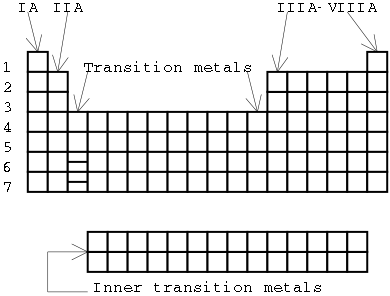
Illustration 1 - Periods and groups of the periodic table.
Introduction
We've discussed how to write electron configurations using the periodic table. We're now going to discuss some of the things that electron configurations can tell you about atoms. Remember that the periodic table is divided into "blocks" based on the electron configuration. So, the properties we discuss will depend on the atom's position in the periodic table - hence the name periodic properties. The properties we will define and discuss in this note pack are:
The atomic radius
The first ionization energy (often just called the ionization energy)
The electron affinity
A review of the arrangement of the periodic table
The periodic table is arranged into periods and groups as illustrated in the following diagram.
|
|
|
Illustration 1 - Periods and groups of the periodic table. |
The horizontal rows on the periodic table are known as periods. The periods on today's periodic table are numbered 1-7. The numerical value of the period is significant. It represents the highest value of the principal quantum number n that the electrons in those elements have. For example, calcium (in period 4) has electrons in the n=4 shell (as well as the n=1, n=2, and n=3 shells), but no electrons in the n=5 shell.
[It took a while to find all the elements we know about today - if you look at older chemistry books, you'll find that many of the elements in period seven will be missing.]
The vertical columns on the periodic table are known as groups. There are several different numbering conventions for the groups. We'll use the older Roman numeral notation because it tells us useful information about the electron configuration. In this notation, the main-group elements are located in the "A" groups and transition elements are located in the "B" groups (not shown on the picture). The main group elements are groups IA - VIIIA (1A through 8A) on the periodic table. These groups include the "s block" and "p block" atoms.
Why this arrangement? The physical and chemical properties of the atoms in each group are similar, because their electron configurations are similar.
The atomic radius and the periodic table
The atomic radius is a measure of the size of the atom. We obviously can't stick a ruler on an atom and measure it from outermost electron to nucleus, as we're not able to determine the exact location of electrons anyway (remember Heisenberg's uncertainty principle). We can , though determine the approximate radius of the atom by seeing where the nuclei of atoms are located in chemical compounds.
Atomic radii of the atoms vary with location on the periodic table as shown. The inner transition metals ("f block") aren't shown.
|
|
|
Illustration 2 - Atomic radius and the periodic table |
Some things to note about the diagram:
The atomic radius increases as you move down a column (down a period).
The atomic radius decreases as you move across a row (across a period).
How can we explain this based on what we know about electron configurations and quantum numbers?
The trend in groups is easy to explain. When we move down a group, we add an entire shell filled with electrons. The outer shell of hydrogen is n = 1, while the outer shell of lithium is n = 2. The lithium atom is larger than the hydrogen atom. The n indicates the energy and approximate size of the orbital an electron occupies.
The trend in periods can be explained by looking at n and the charge on the nucleus. When we move across the periodic table, we increase the change on the nucleus (atomic number goes up!). We're not increasing the space available for electrons by adding new shells, though. We're also not adding more electrons in the inner shells to "shield" the nucleus. An outer shell electron in neon, for example, sees the nucleus as well as an outer shell electron in lithium, but neon's nucleus has a +10 charge rather than a +3 charge. As a result, neon's outer shell electrons are pulled closer to the nucleus than lithium's.
This trend isn't perfect,, but it works very well for main-group elements.
Ionization energy and the periodic table
Most elements are stable species. Many elements can exist in uncombined form, even though they can react fairly easily with other elements. Magnesium is a good example of this. We know that magnesium will rapidly burn if heated in air to form the compound magnesium oxide, and that this reaction is very exothermic. Why doesn't a piece of magnesium spontaneously burst into flames while sitting on the table?
Part of the answer to this question involves the ionization energy of magnesium, expressed in kJ/mol units. The first ionization energy is the amount of energy required to remove the outermost electron (the highest energy electron) from an atom. Magnesium has an electron configuration of 1s22s22p63s2. The ionization energy is the energy required to remove one of the 3s2 electrons (leaving magnesium with a +1 charge and the electron configuration 1s22s22p63s1). The higher the ionization energy of an element, the more difficult it is to remove an electron from that element. In magnesium's case, you must provide enough energy to remove electrons from magnesium atoms before they will react.
Ionization energies also vary with the atom's location on the periodic table, as shown below.
|
|
|
Illustration 3 - Ionization energy and the periodic table |
Things to note:
The ionization energy decreases as you move down a column (down a group).
The ionization energy increases as you move across a row (across a column).
So, how can we reconcile this with what we know about atoms and quantum numbers?
As before, the trend in groups is easy to understand. We know that charge interaction decreases with distance between the charges. As we move down a group, the outermost electron is in a shell with a higher value of n. In other words, it's farther away and less attracted to the nucleus. It is easier to remove an electron from an atom of potassium (1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 ) than an atom of lithium (1s 2 2s 1 ). Potassium's 4s electron comes off easier than lithium's 2s electron because it's much farther away from the nucleus and it's shielded from the charge of the nucleus by more electrons. This is one reason that potassium reacts more vigorously than lithium - it requires less energy to kick off the reaction.
The trend in periods can be explained by looking at the atomic radius and remembering what we said about charge interactions and distance. As we move across a period, the atoms get smaller. Electrons are held more closely to the nucleus, so it takes more energy to remove the electron. It takes much more energy, for example, to remove an electron from neon than it does from lithium. Neon's outermost electrons are held more closely than lithium's.
Ionization energy is particularly important in the formation of ionic compounds. You can remove more than one electron from an atom by adding more energy. It becomes progressively more difficult to remove valence electrons and becomes very difficult to remove core electrons (electrons which are located in the inner shells of the atom). Magnesium typically loses its two valence electrons in forming ionic compounds, but it does not lose its core electrons.
Electron affinity and the periodic table
The ionization energy deals with the formation of cations (positively charged ions). There is a property related to anion formation (negatively charged ions), and it's called the electron affinity. The electron affinity is the energy change on adding an electron to an atom, and is usually expressed in units of kJ/mol. The sign of the electron affinity is usually negative, signifying that energy is released when an electron is added to an atom.
The more negative the electron affinity is, the more stable the anion formed.
There is a rough periodic trend here. It's not as easy to spot as the other periodic trends.
To summarize:
In general, the electron affinity becomes more negative as you move across a period, with exceptions.
Groups IIA and VIIIA do not form stable anions.
Group VA does not follow the trend above.
We can explain the exceptions
Filled subshells (like the "s" subshells in a group IIA atom) are more stable than unfilled subshells, so groups IIA and VIIIA are reluctant to start a new subshell.
Half-filled subshells are slightly more stable than other configurations (except filled), so group VA is reluctant to add another electron.
Electron affinity influences both the formation of ions in the nonmetals and the formation of molecular compounds.
Summary
We've discussed the layout of the periodic table and how it can be used to estimate several properties of atoms based merely on their location in the periodic table. These properties were the atomic radius, ionization energy, and the electron affinity. We defined each one of these properties and explained their trends in terms of what we understand about the atom and quantum mechanics. These properties are important to explain chemical bonding.
All original site content ©2007 Charles Taylor. Page updated: November 28, 2007.