Illustration 2 - Graphic of a modern periodic table
Introduction
The periodic table is probably the most popular graphic from the world of chemistry - visible on T-shirts, in video games, and occasionally on album covers. Other than the atomic symbols of a few of the elements and the chemical formula for water, the table is one of the only things that people who have never had any training in science at all will recognize. As a beginning chemist, it isn't enough for you to merely recognize the table - you should understand a little about its history and understand the features of the periodic table that make it useful to chemists.
History of the table
The concepts behind the periodic table date back to the 1800s. Dalton's atomic theory gave early chemists a unifying framework for studying the elements. Based on the theory, it was possible to determine (by setting an arbitrary value for one atomic weight), the relative atomic weights of elements that had been discovered. After sufficient weights had been determined, scientists like Mendeleev and Meyer observed that, when the elements were arranged in order of increasing atomic number, the properties of the elements would repeat at regular intervals. More than that, it was possible to calculate the physical properties of elements that had not yet been isolated, since the physical properties of the elements appeared to be a function of the atomic weight.
These observations were the foundation of the periodic law , which said that the properties of elements were periodic functions of their atomic weights . The known elements were arranged into several groups , and elements in each group were physically and chemically similar to each other. These groups were often presented in table format, giving rise to the early periodic table of the elements . An early periodic table is reproduced below.
Illustration 1: A periodic table from 1900
|
|
Group I |
Group II |
Group III |
Group IV |
Group V |
Group VI |
Group VII |
Group VIII |
|---|---|---|---|---|---|---|---|---|
|
Period I |
Li, 7 |
Be, 9 |
B, 11 |
C, 12 |
N, 14 |
O, 16 |
F, 19 |
|
|
Period II |
Na, 23 |
Mg, 24 |
Al, 27 |
Si, 28 |
P, 31 |
S, 32 |
Cl, 35.4 |
|
|
Period III |
K, 39 Cu, 63 |
Ca, 40 Zn, 65 |
Sc, 44 Ga, 70 |
Ti, 48 Ge, 72 |
V, 51 As, 75 |
Cr, 52 Se, 79 |
Mn, 55 Br, 80 |
Fe, 56 / Ni, 59 / Co, 59 |
|
Period IV |
Rb, 85 Ag, 108 |
Sr, 87 Cd, 112 |
Y, 89 In, 114 |
Zr, 90 Sn, 118 |
Nb, 94 Sb, 120 |
Mo, 96 Te, 127 |
???, 100 I, 126.5 |
Ru,102 / Rh,103 / Pd, 106 |
|
Period V |
Cs, 133 ??? ??? Au, 197 |
Ba, 127 ??? ??? Hg, 200 |
La, 138 ??? Yb, 173 Tl, 204 |
Ce, 140 ??? ??? Pb, 207 |
Pr, 140 / Nd, 144 ??? Ta, 183 Bi, 208 |
??? ??? W, 184 ??? |
Sm, 150 ??? ??? ??? |
??? Os, 191/ Ir, 193 / Pt, 195 |
|
|
|
|
|
Th, 232 |
|
U, 239 |
|
|
This periodic table is a reproduction of the periodic table found on page 246 of Richter's Inorganic Chemistry (5th American edition, translated by E. F. Smith). This book was published in 1900, and gives a good account of the state of the periodic table in that year.
The overall organization of the table is preserved in the modern periodic table. Groups, composed of chemically or physically similar elements, are the columns. Periods are rows.
The question marks in the periodic table represent elements that scientists believed should exist, but had not yet been isolated (in particular, notice the lack of noble gases like argon, neon, helium, and krypton. Some noble gases had first been isolated within a year or two of the publication of this table). Many of these blanks would be filled in during the early decades of the 1900s.
From the early table to the modern table
There were some problems with the old periodic law. These were known even in the 1900s, but weren't solved until more became known about the internal structure of the atom. Probably the most troublesome to early chemists was the problem with tellurium and iodine. In the table reproduced above, tellurium's weight is given at 127, while iodine's is given as 126.5. Tellurium is heavier than iodine, but has the chemical properties of the Group VI elements. Iodine is lighter than tellurium, but is more similar to Group VII elements. In short, these elements are backwards from the order predicted by the old periodic law.
Initially the iodine/tellurium problem was attributed to faulty measurements of the atomic weight of tellurium. These measurements were relatively new and difficult to perform, and the not-too-unreasonable hope was that as the measurements got better, the problem would go away. Repeated measurements, however, only confirmed the problem. It took insight into atomic structure that came with modern chemistry to solve this problem, and the periodic law was rewritten as a result of it.
The main thing that separates the old periodic table from the new is the way the elements are organized. We now know that the properties of the atoms are periodic functions of the atomic number , rather than the atomic weight . New periodic tables organize the elements by atomic number. When organized by atomic number instead of atomic weight, tellurium and iodine naturally fall into the correct groups. (So why did early chemists use the atomic weight? First, the atomic weight usually gave the correct order. Second, the atomic weight was the only number the early chemists had. The atomic number hadn't been discovered yet!)
The modern periodic table
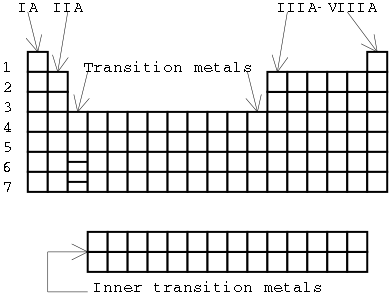
A modern periodic table looks like this.
|
|
|
Illustration 2 - Graphic of a modern periodic table |
The modern periodic table is organized into groups and periods much like the old periodic table. You'll also find the symbols and atomic weights of each element listed. In addition, you will find the atomic number of each element. On a few tables, you may also find information about the structure of the electron cloud of each element.
There are two numbering systems for groups. One of them uses a system of Roman numerals that is similar to the old periodic tables, and the other uses a system that simply numbers the groups from 1 to 18 (not shown in the illustration). We will use the system involving Roman numerals in this class. The "A" groups are called the main group elements, and will be our primary focus in this course. The transition metals or "B" groups are quite similar to each other in terms of overa ll properties. The main groups, though, vary quite a bit. For example, Group IA and Group VIIIA are very different in properties, while groups IB and VIIIB are quite similar to each other. The inner transition metals actually belong where the extra lines have been placed in periods 6 and 7, but they are usually written below the table. This helps the periodic table fit on a conveniently-sized piece of paper.
Periods are simply numbered from 1 to 7. If more elements are discovered, we may add an eighth period. (We haven't actually finished filling in all of period 7 yet, since many of these elements are very unstable.)
A typical "block" of the periodic table looks like this.
|
|
|
|
Illustration 3 - Sodium, as depicted on a modern periodic table. |
|
Properties of the elements and the periodic tables
Most of the elements are solids at room temperature - particularly as you go down and to the left on the periodic table. Most of the elements that normally exist as a gas at room temperature are on the upper right. There are also two elements that are liquid at room temperature (mercury and bromine). One element is a solid at room temperature, but will readily melt in your hand (gallium).
The elements can be grouped into three broad categories based on their physical and chemical properties: metals , nonmetals , and metalloids (also known as semimetals or semiconductors ).
The largest category in terms of sheer number of elements is the metals . The metals have certain properties in common:
Most metals are solid at room temperature. The only known exception is mercury.
Metals conduct electricity in the solid and liquid states. Some metals conduct better than others, but all are conductors.
Metals are good conductors of heat.
Metals are malleable. In other words, they can be shaped by hammering.
Metals are ductile. In other words, they may be drawn out into wires.
Metals have a characteristic metallic sheen. Metals can be different colors (compare gold, silver, copper, and lead), but all have a similar shininess.
On the periodic table, metals are found almost all the way across the table except for the far right and the very upper left-hand corner (hydrogen).
|
|
|
Illustration 4 - The location of the metals on the periodic table |
The metals are shaded gray in the illustration above. Elements that have not yet been isolated have been removed from the table.
The second-largest group of elements, at least in terms of number of elements, are the nonmetals . The nonmetals have (as you'd expect by the name) characteristics that are nearly the opposite of the metals.
Eleven of the known nonmetals are gases at room temperature. The others are mostly solids, except bromine (a liquid). On average, then, the nonmetals are less dense than the metals.
Nonmetals are not good conductors of either heat or electricity.
Metals are neither malleable nor ductile. Most solid nonmetals are brittle.
On the periodic table, you can find nonmetals clustered on the right-hand side of the periodic table (with the exception of hydrogen, which is usually placed at the upper left).
|
|
|
Illustration 5 - The location of the nonmetals on the periodic table |
The nonmetals are marked in gray on the illustration above.
The third and smallest group of elements on the periodic table is the metalloids. The metalloids are an odd bunch. They exhibit some properties of both the metals and nonmetals. For example, metalloids are poorer conductors of electricity than the metals, but are better conductors than nonmetals. When metalloids are mixed with other elements, some interesting effects can be observed - especially regarding electrical conductivity. The metalloids and their unique properties make modern computers and electronics possible.
The metalloids are between the metals and nonmetals on the periodic table - just like they are in their overall properties.
|
|
|
Illustration 6 - The location of the metalloids on the periodic table |
The metalloids are shown in gray in the illustration above. The thick zig-zag line that the metalloids are clustered around is often drawn on periodic tables as an aid to remembering where the metalloids are located.
Summary
In this note pack, we have discussed some of the the history of the periodic table and compared the early periodic table with the modern version. We have also discussed the important features of the periodic table and where the different kinds of elements are located. You should be able to work with the modern periodic table and pick out information like group number, period number, atomic weight, and atomic number. You should also be able to discuss the properties of metals, nonmetals, and metalloids and identify them by their location on the periodic table.
All original site content ©2007 Charles Taylor. Page updated: November 28, 2007.