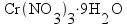
CaCl2
CCl4
Fe(NO3)3
FeF2
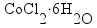
BaO
CuS
NH4I
Cd(HCO3)2
|
CHM 110 |
|
|
Nomenclature Practice |
|
|
|
|
|
|
|
Write the chemical formulas of the following compounds.
|
sodium chloride |
|
|
magnesium fluoride |
|
|
iron(III) sulfate |
|
|
titanium(IV) nitride |
|
|
hydrochloric acid |
|
|
ammonium sulfate |
|
|
copper(I) chloride |
|
|
lithium carbonate |
|
|
sulfur dioxide |
|
|
dihydrogen monoxide |
|
|
calcium hydroxide |
|
|
diphosphorous pentoxide |
|
|
phosphorous trichloride |
|
|
potassium carbonate |
|
|
nickel(II) nitrate hexahydrate |
|
Write the name of the following compounds
|
|
|
|
CaCl2 |
|
|
CCl4 |
|
|
Fe(NO3)3 |
|
|
FeF2 |
|
|
|
|
|
BaO |
|
|
CuS |
|
|
NH4I |
|
|
Cd(HCO3)2 |
|
All original site content ©2007 Charles Taylor. Page updated: November 28, 2007.