Electron Configuration
Introduction
We previously discussed quantum theory
along with atomic orbitals and their shapes. We're still missing
some pieces of the puzzle, though. How do electrons in an atom
actually occupy the atomic orbitals. How does this arrangement
affect the properties of an atom and the ways atoms bond with each
other?
We will now discuss how an atom's
electrons are arranged in atomic orbitals. Knowing a bit about this
might help us figure out how atoms bond.
The Pauli Exclusion Principle
We have described (and drawn) atomic
orbitals. But how many electrons can an atomic orbital hold? The
answer lies in the quantum numbers, specifically the fourth
quantum number. The first three quantum numbers (the principal
quantum number, the angular momentum quantum number, and the magnetic
quantum number) select the atomic orbital. For example, let's look
at a set of quantum numbers of an electron:
n = 2 ; l = 1 ; ml
= 0
What do these three quantum numbers
tell us about this electron? We're told that the electron is in the
second energy level (n = 2), that the electron is in one of
the "p" orbitals (l
= 1), and we're told the orientation of the "p" orbital the
electron is in. But to specify the exact electron, we need the
fourth quantum number- the spin
quantum number . Recall
that this quantum number can have only two values (+½ and -½)
and that no two electrons in the same atom can have the same set of
four quantum numbers. So, the orbital defined above can have
at most two electrons. The observation that no two electrons in an
atom can have the same four quantum numbers is called the Pauli
Exclusion Principle .
If you look at how many electrons a
subshell can hold (subshells are represented by l
values):
For
a "p" subshell, l
= 1. This means that possible values for m l
are -1, 0, and 1. m s
can have two values, so the maximum number of electrons in a "p"
subshell is six
- two electrons for each possible value of ml.
For a "d" subshell, the
maximum number of electrons is ten.
For a "f" subshell, the
maximum number of electrons is fourteen .
See if you can verify these last two on your own.
Orbital diagrams
We represent the arrangement of
electrons in an atom (the electron configuration) using an
orbital diagram, a picture that shows the arrangement by
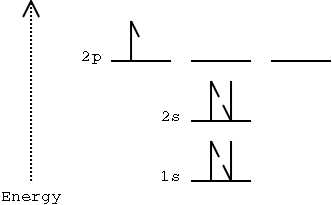
orbital. A sample orbital diagram for the boron atom is shown below.
Boron has five protons and five electrons.
|
|
Electrons are represented by arrows.
The electron spin (the magnetic quantum number) is
represented by the direction of the arrow, up or down.
ml is represented by the blanks (note
that there are three blanks for the 2p subshell).
Two electrons with the same spin can't be in the same
orbital.
The orbitals fill by energy - lower energy orbitals fill
first.
|
|
Illustration 1 - Atomic orbital diagram for boron
|
|
A shorthand way to describe the
arrangement of electrons (instead of drawing the diagram) is to just
write the electron configuration using the subshell name with a
superscript containing the number of electrons in the subshell. For
example, we would write boron 's
electron configuration as :
B: 1s22s22p1
As a further way to cut down the length
of an electron configuration, we can use noble gas core
notation. Atoms in group VIIIA on the periodic table are
called noble gases (He, Ne, Ar, Kr, Xe, Rn). The electron
configuration of helium, for example is:
He: 1s2
We can shorten the amount of writing we
have to do by picking the noble gas with atomic number nearest but
not above the atom we're writing about and writing it in
brackets. So, we could write boron's electron configuration as
B: [He]2s22p1
For boron, this doesn't shorten things
much, but for a large atom like cesium (with 55 electrons), this
reduces the amount we have to write considerably!
The valence electrons
Why would we want to shorten the
electron configuration using the noble gas core notation?
Aren't those electrons we're omitting important?
To us, the most important electrons in
the atom are the electrons in the outer shell (the highest
value of n). These electrons are commonly called the valence
electrons, and they are the electrons that usually participate in
chemical reactions.
Why are these the electrons that react?
The simple answer is that they're the ones on the outside of the
atom. When two atoms come together, the electrons that see each
other first are the valence electrons- the ones on the outside.
So, boron's valence electrons are the
two electrons in its "2s" orbital and the electron in the
"2p" orbitals. (2s22p1). The two
electrons in boron's 1s orbital (1s2) are buried too deep
inside the atom to react.
Writing electron configurations of
atoms - the periodic table
You might be wondering at this point
how you write an electron configuration - how to determine the order
that orbitals fill up in. We said when talking about orbital
diagrams the orbitals fill up from lowest energy to highest energy.
You can probably see a pattern to this already - lower "n"
orbitals generally fill up before higher "n" orbitals -
because they have lower energy than the higher orbitals. "s"
orbitals generally fill up before "p" orbitals, which fill
up before "d" orbitals, etc.
In fact, if you have a periodic table
handy, it's very easy to write an electron configuration. Elements
are arranged on the periodic table in groups which have similar
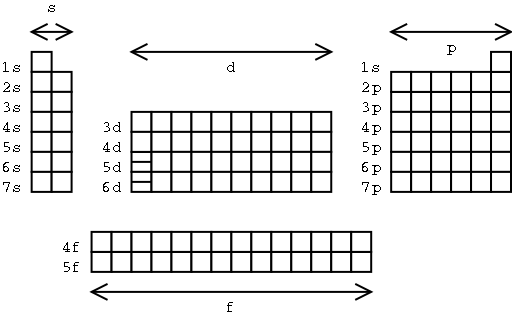
electron configurations. This is illustrated on the exploded
periodic table below.
|
|
|
Illustration 2 - The "blocks" of the periodic table
|
A few notes on this diagram:
An element's position on the table
indicates the last subshell being filled with electrons.
The "s block" is two
elements wide, the "p block" is six elements wide, the "d
block" is ten elements wide, and the "f block" is 14
elements wide. Where have you seen these numbers before?
Helium. By convention it's
written at the far right of the periodic table with the other noble
gases. Its electron configuration is 1s2, not
1s11p1 (which isn't allowed by quantum
mechanics).
The d and f
subshells are particularly energetic - more so than nearby s
subshells with higher values of n! (For example, 3d fills
only after 4s does.)
The "f block" elements
at the bottom of the table are inserted after the two elements with
the extra lines in the "d block".
So, let's look at a few electron
configurations:
Mg: 1s22s22p63s2
OR [Ne]3s2
You can trace down the periodic
table with your finger to write this - start with element 1
(hydrogen) and go across each element, adding electrons to your
configuration until you get to your element.
The ideal gas core notation
shows the valence electrons clearly in this example.
Fe: 1s22s22p63s23p64s23d6
OR [Ar]4s23d6
Note that the "4s"
subshell fills before the "3d". This is because "d"
orbitals have a high energy and the "3d" orbitals actually
have a slightly higher energy than the "4s" orbitals. The
"3d" electrons are not considered valence electrons,
though they do play a role in some of iron's more interesting
chemical reactions.
There are a few exceptions to our way
to generate electron configurations from the periodic table, mainly
in the transition metals, but we'll not concern ourselves much with
them. They relate to the observation that filled and half-filled
subshells are more stable (lower energy) than other states and are
mainly found in a few of the "d block" and "f block"
elements.
Electron configurations, Hund's
rule, and magnetism
We've discussed the order in which the
subshells fill up - but we need to talk a bit about the order
in which the orbitals fill. There are three "p"
orbitals within any "p" subshell, five "d"
orbitals in any "d" subshell, etc. How do these orbitals
fill up, and is there any evidence that these orbitals fill up in the
way we say they do?
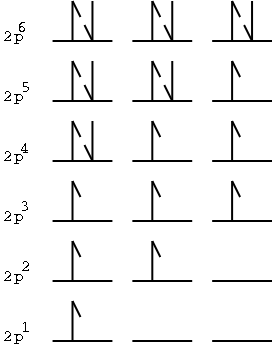
This question is answered by Hund's
rule - which says that the lowest-energy arrangement of electrons in
a subshell is obtained by filling orbitals with electrons of the same
spin before pairing.
So, the "p" orbitals fill up
like this:
|
|
|
|
Illustration 3 - Hund's rule
|
|
There is experimental evidence for
Hund's rule. Spinning electrons are magnetic, but opposite spins
cancel. So, Hund's rule predicts which elements are magnetic (those
having unpaired electrons). Elements with unpaired electrons are
paramagnetic - that is, they're attracted to a weak magnetic
field. Atoms with no unpaired electrons are diamagnetic
- they don't interact at all or they're repelled by the field.
Elements predicted by Hund's rule to be paramagnetic have been
experimentally found to be paramagnetic. [Paramagnetism isn't the
same thing as the magnetism you may have been exposed to in an
introductory physics course - like from an iron magnet.
Paramagnetism is a much weaker effect and is not limited to certain
metals.]
Summary
This note pack
discussed electron configurations - you learned to write electron
configurations for atoms using both the rule of electrons filling
orbitals from lowest to highest or from using the "blocks"
of the periodic table. Next, we'll talk more about the periodic
table and take a brief tour of properties predicted by an element's
electron configuration.
All original site content ©2007 Charles Taylor. Page updated: November 28, 2007.


