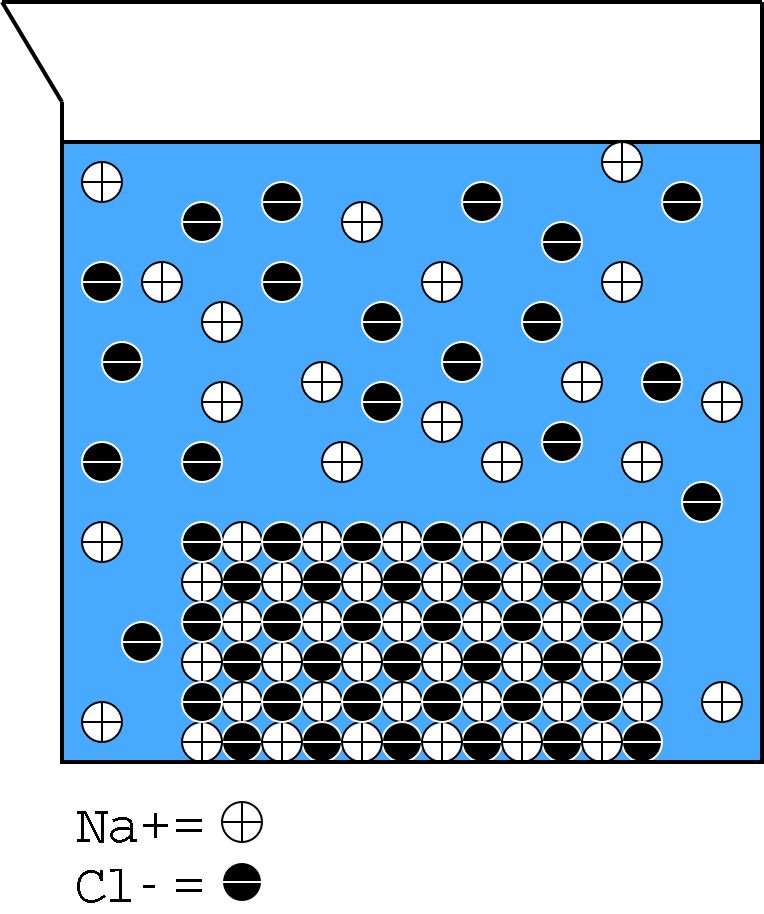
Sodium and chloride ions are distributed throughout the solution.
This solution will conduct electricity.
Illustration 1 - NaCl solution
Introduction
In 1884, Arrhenius proposed that some substances broke up when dissolved in water to form freely moving ions . We've already defined ions as charged s pecies present in ionic substances. We'll now look at them as species that are able to carry charge from one point to another - in other words, we'll look briefly at ions as conductors of electricity.
Charge-conducting solutions (solutions containing ions) are important in industrial processes and everyday life. For example, the liquid in a lead-acid battery like the one in your car is a charge-conducting solution.
Electrolytes
Some species will dissolve to form solutions that can conduct current. These are called electrolytes. Others do not, and these are called nonelectrolytes.
Examples:
|
Electrolytes |
Nonelectrolytes |
|---|---|
|
Table salt (NaCl) |
Pure H2O |
|
Acetic acid (HC2H3O2) |
Sugar (C12H22O11) |
Chemically:
|
Chemical Reaction |
Notes |
|---|---|
|
NaCl(s) --> Na+(aq) + Cl-(aq) |
Ionic solutions contain ions which can carry charge. |
|
C12H22O11(s) --> C12H22O11(aq) |
Uncharged molecules are unable to carry a charge in solution. |
What makes an electrolyte conduct?
An electric current is caused by the motion of charged particles. For a current to flow in any material, you must have charge carriers - species that are themselves charged. In a solution, these would be ions. For an electric current to flow, these ions must be free to move about in the solution.
Illustration 1 shows a cartoon drawing of a sodium chloride crystal dissolving in solution. When sodium chloride dissolves, sodium cations and chloride anions are spread throughout the solution. These free ions can move from one side of a solution to the other, allowing a current to flow.
|
|
|
|
Illustration 1 - NaCl solution |
|
Illustration 2 is another cartoon illustration, this time of a crystal of table sugar (C12H22O11) dissolving into solution. While sugar molecules do spread out into the solution as sodium and chloride ions would, sugar molecules do not carry an overall charge. The sugar molecules can move from one side of the solution to the other, but they are uncharged so they cannot carry an electric current.
|
|
|
|
Illustration 2 - C12H22O11 solution |
|
Electrolytes vs nonelectrolytes
How can we tell electrolytes from nonelectrolytes without the experimental apparatus? We can look at the chemical formula of the compound.
If a compound is ionic and can be made to dissolve in water, it is an electrolyte.
If a compound is molecular and can be made to dissolve in water, it may or may not be an electrolyte. Acids and bases are electrolytes, since they form ions when dissolved in water.
Examples:
|
Compound |
Class |
Dissolves in water? |
Electrolyte? |
|---|---|---|---|
|
NaCl |
ionic |
yes |
yes |
|
HCl |
molecular |
yes |
yes |
|
HC2H3O2 (Acetic acid) |
molecular |
yes |
yes |
|
CH3CH2OH (Ethanol) |
molecular |
yes |
no (nonelectrolyte) |
|
C12H22O11 (Sugar) |
molecular |
yes |
no (nonelecrolyte) |
Strong vs weak electrolytes
You might wonder whether some solutions are better at conducting charge than others. You'd be right if you thought that some substances form solutions that carry charge very well and some substances form solutions that, while they do carry charge, aren't that good at it. Acetic acid, for example,, will form a charge-carrying solution in water. A solution of an equivalent amount of sodium chloride, though, will carry charge much better than the acetic acid solution. The ability of a solution to carry charge is directly related to how many ions (the charge carriers) are present. Sodium chloride, apparently, forms more ions than acetic acid does.
You know that there are substances that don't ionize at all and that there are other substances that do ionize. It's reasonable to suppose that there are some substances which ionize only to a small degree when dissolved in water. These substances are called weak electrolytes. Acetic acid (HC2H3O2) falls in this class. Sodium chloride, on the other hand, is a strong electrolyte. Strong electrolytes ionize almost completely in aqueous solution and form many more charge carrying ions than weak electrolytes do. Solutions of strong electrolytes tend to be very good conductors of electricity.
How do we tell strong electrolytes from weak using only a chemical formula rather than a testing apparatus? Here are some hints.
Molecular substances that are electrolytes tend to be weak electrolytes. (There are a some exceptions - the "strong acids" like HCl, HNO3, and H2SO4).
Ionic substances that dissolve in water are strong electrolytes.
Solubility and the solubility rules
In our discussion on electrolytes, we said that ionic substances "that dissolve in water" are electrolytes. Not all ionic substances dissolve readily in water. You can see this easily in the lab. One of your early labs involved the mixing of solutions of different chemicals together. One of the signs a chemical reaction occurred was precipitation (a substance which doesn't dissolve in water is formed from two substances that do). These precipitates were ionic compounds that don't dissolve in water.
In the language of chemistry, compounds that readily dissolve in water (like NaCl) are said to be soluble. Those that do not readily dissolve in water (such as AgCl) are said to be insoluble.
Many experiments have been conducted on the solubility of ionic compounds, and luckily for us most of these experiments can be summed up in a few simple rules (which you will need to memorize).
The first few rules tell what compounds are soluble. These compounds will dissolve readily in water.
|
Soluble compounds |
Exceptions |
|
1) Group I A and NH4+ compounds
|
None |
|
2) Acetates (C2H3O2-) and nitrates (NO3-)
|
None |
|
3) Chlorides (Cl-), bromides (Br-), and iodides (I-)
|
Silver, mercury, and lead. |
|
4) Sulfates (SO42-)
|
Silver, mercury, lead, calcium, strontium, and barium. |
The last few rules tell what compounds are insoluble. These compounds will not dissolve readily in water.
|
Insoluble compounds |
Exceptions |
|
5) Carbonates (CO32-), phosphates (PO43-), and sulfides (S2-)
|
Group I A and NH4+ compounds |
|
6) Hydroxides (OH-)
|
Group I A and NH4+ compounds, calcium, strontium, and barium |
So, soluble ionic compounds will dissolve in water to form solutions that are strong electrolytes. Insoluble ionic compounds won't dissolve in water, so they don't form solutions that conduct electricity.
Summary
In this note pack, we have discussed Arrhenius's ionic theory of solutions (which separates substances into electrolytes and nonelectrolytes). We have discussed how to differentiate, given a chemical formula, between electrolytes and nonelectrolytes. We then discussed solubility and the so-called "solubility rules". These rules will allow you to decide whether an ionic compound is water-soluble (and therefore an electrolyte) based on its chemical formula. You should memorize these rules, as they will also be useful for determining the identity of precipitates formed in chemical reactions.
All original site content ©2007 Charles Taylor. Page updated: November 28, 2007.