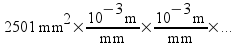
Introduction
This note pack will give you a strategy for dealing with dimensional analysis problems. Since the first application of dimensional analysis you learned was unit conversions, we will focus on those for now.
Why?
You won't get far with dimensional analysis if you don't have a plan of attack for solving the problems. From previous years, I've noticed that people who don't have a plan for solving these problems will get them right only about half of the time! Unless you want to make 50s on your tests, you need a plan of attack.
The plan - with an example
The problem: Convert 2501 mm2 to cm2.
This is a multiple-step dimensional analysis. In other words, you need more than one conversion factor to solve the problem.
Step 1: Write out the conversion factors.
This is an absolutely critical step to successfully solve any dimensional analysis problem. Don't skip it.
Really. Write out the conversion factors!
In a simple metric units problem, the conversion factors are generated from the metric prefixes. So, your first step is to write out any prefixes you see in the problem statement. In this problem, the prefixes are:
c = 10-2
m = 10-3
Then make conversion factors by multiplying the base unit you're working with by both sides of the equations we wrote above. In this case, the base units are meters. (Note that m2 is not a base unit!)
cm = 10-2m
mm = 10-3m
Step 2 : Write down the starting number (with units), then multiply by a conversion factor that will cancel out its units. When writing the factor, write the part of the factor containing the unit you don't want on the bottom, and write the other part of the factor on the top.
In our problem, we start with 2501 mm2...
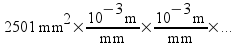
We want to get rid of the mm2 units, so we arrange our conversion factor so that millimeters (mm) is in the denominator. This forces us to put the equivalent of a millimeter (10-3 m) in the numerator. The undesired unit is canceled out.
So why did we multiply twice? Simple! Remember that we're dealing with 2501 square millimeters (in other words, millimeters times millimeters), so we had to cancel out both of them.
Step 3: Multiply by a conversion factor that will cancel out any remaining units, leaving you with your desired unit. Again, write the part of the factor containing the unit you don't want on the bottom, and write the other part of the factor on the top.
Continuing with our example, step 2 left us with our number in square meters, but we want to convert it to square centimeters. We use our other conversion factor - which will convert from meters to centimeters.
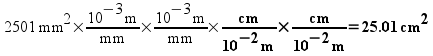
Problem solved!
Potholes to avoid
Here are a few simple (but common) mistakes that you can avoid easily:
Putting the number (for example, the 10-3 in our example) in the wrong place : Avoid this by remembering to write out your conversion factors . The number always goes with the unit it is with in the conversion factor equation.
Calculator errors: Avoid this by learning how to enter numbers in scientific notation on your calculator. "10-3", for example can be entered into most calculators as "E-3" or "1E-3". (Your calculator may have "EE" or "EEX" - see me if you're not sure how to enter scientific notation into your calculator.).
Multiplying by the wrong thing: Avoid this by always checking to make sure your units cancel out properly and the only thing left is the unit you want.
The plan in brief
Write out your conversion factors. If metric prefixes are involved, generate conversion factors from prefixes.
Multiply the original number by a conversion factor to cancel out its units. Put what you don't want on the bottom. For simple one-step problems you're done after you multiply by the first factor.
Multiply by additional conversion factors until all units but the desired unit have been canceled out.
Summary
In this note pack, you have been given a three -step problem-solving strategy that will let you easily solve dimensional analysis problems. This note pack will do you little good, however, unless you practice. Try problems until you're confident that you can do these conversions reliably. We won't be doing many metric unit conversions later in the course, but we will use the method of dimensional analysis to solve chemical problems. The method is the same as outlined here, but the conversion factors will come from other places - like chemical equations.
All original site content ©2007 Charles Taylor. Page updated: November 28, 2007.