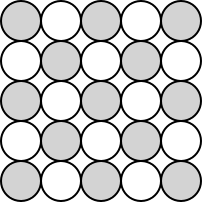
Dalton said that you couldn't break down atoms.
Dalton didn't propose anything at all below the level of the atom.
Illustration 1 - Dalton's picture of atoms
Introduction
It is important that we know how substances are put together. The way things are put together influences their characteristics, much like the construction of a bridge influences the load it can carry. As chemists, we are interested in the way that atoms and molecules are put together, because this influences how they react in chemical reactions. We'll start our discussion with the atom, as it's the basic building block of the chemical substances we are familiar with.
History: Older conceptions of the atom
We discussed some early conceptions of matter back in the second chapter. The first of these was Dalton's atomic theory. Dalton said that matter was composed of indivisible particles which he called atoms. He didn't speculate about any kind of internal structure the atom might have. After all, why bother inventing a structure for something that you are unable to break down? So Dalton's picture was much like this:
|
|
|
|
Illustration 1 - Dalton's picture of atoms |
|
In the illustration, you see how Dalton might have envisioned a compound. The shaded circles are atoms of one element, while the white circles are atoms of another element. Note that there's no other structure proposed.
Later, a better model of the atom was developed. This new model was called the nuclear model. Rutherford's gold foil experiment (and others) showed that there was indeed structure to the atom - a central, dense nucleus that was positively charged and a diffuse "cloud" of negatively charged electrons surrounding it and making the overall atom electrically neutral. The nuclear model gives us detail on the inside of the atom.
|
|
|
|
Illustration 2 - The nuclear model of atoms |
|
The nuclear model was much more informative than Dalton's indivisible atoms were - it told us something about how the inside of an atom is arranged. However, there were many things lacking from this model, such as:
How are the electrons distributed in the "cloud"?
Why don't electrons fall into the nucleus? (In other words, why is the atom stable?)
How can we predict chemical reactivity based on the structure of the atom?
There were other important properties that the nuclear model failed to predict. One of these properties - the behavior of atoms when excited enough to give off light energy - was used to come up with a new model of atomic structure.
Toward a more modern conception of the atom: Atomic line spectra
When atoms are excited (by adding energy to them), they can give off light. A good example is the streetlight. Older streetlights use mercury vapor lamps, which give off a blue light. Newer streetlights use sodium vapor lamps, which give off an orange light. These lamps contain atoms which are excited by applied electricity. You can observe an interesting phenomenon if you look at lights like these through a prism.
|
|
|
Illustration 3 - Separation of light from excited element by a prism |
The light is separated into a few distinct colors, rather than into a rainbow. This is called a line spectrum, because only a few "lines" of certain colors are emitted. Furthermore, if you were to switch elements, you would get a completely different pattern. These atomic line spectra are in essence the "fingerprints" of the atoms.
How did the nuclear model deal with atomic line spectra ? It didn't. These atomic fingerprints are related to the internal structure of the atom - the structure of the electron cloud that the nuclear model does not reveal.
The Bohr model of the atom
Niels Bohr, in the early 1900s, came up with a new theory of atomic structure which could explain atomic line structure. He attempted to do what the nuclear model failed to do and describe how electrons were arranged in the atom. Bohr proposed some rules for the places in an atom where electrons were allowed to be (and backed it up with mathematics which we won't get into).
Electrons can have only specific energies in an atom. These were called energy levels , and were related to the distance the electron was from the nucleus.
Electrons can change energies only by changing from one discrete energy level to another. These changes in energy level were called transitions.
Bohr developed his theory based on earlier work that showed that energy was quantized , that is, it could only be transmitted in "packets" of at least a certain amount. (If you're familiar with computer networks, they too transmit data in packets of certain sizes!) Bohr reasoned that electron energies, too, followed this pattern . If you envision the energy levels of electrons as the distance of the electrons from the nucleus (an approximation, but a good place to start), you can picture the atom like this:
|
|
|
|
Illustration 4 - Energy levels and transitions |
|
You can see how Bohr's model explains line spectra. Electrons can only have certain energies and can only gain or lose certain amounts of energy by changing energy levels. So, atoms will only emit light at certain frequencies (or wavelengths) when their electrons change energy levels. Different elements have different arrangements of electrons, so their "fingerprints" will be different.
The electron on the right relates to one of the labs you will perform - absorption of light by atoms. Electrons in an atom can absorb light, but only if the photon has the correct energy to move the electron up one or more energy levels. Otherwise, the photon is not absorbed. This is evident in our experiment - there is a certain wavelength where maximum absorbance of light takes place. Other wavelengths allow the light to pass through without being absorbed.
Bohr was even able to calculate from his theory the (correct) atomic line spectrum for the hydrogen atom.
Unfortunately, Bohr's model accurately predicted the atomic line spectra for only the hydrogen atom. For other atoms, the transitions occurred at other energies than predicted by the Bohr model. This meant that the Bohr model, while an important step forward over earlier models, was not complete. Since hydrogen only has one electron and other atoms have more, the logical place to start to improve the Bohr model was to look for interactions between electrons in multi-electron atoms.
Explanations for the line structure and most other observable characteristics of multi-electron elements are provided by a quantum mechanical model. Quantum mechanics is the branch of science that deals with motion of extremely small objects (like electrons). Classical mechanics or Newtonian mechanics is the branch of science that deals with the motion of large objects, and is the kind of mechanics that you have already been exposed to in high school. Classical mechanics does not work for extremely small objects, so quantum mechanics was invented.
Summary
We've reviewed the old theories of atomic structure - focusing on where they put the electrons. We've discussed the limitations of each of the old models, and explained how the newer models described atomic properties better than the old. At this point, we've discussed the Bohr model and the fact that it was the first to establish why there were such things at atomic line spectra at all. We'll soon study how quantum mechanics improves further our picture of the atom.
All original site content ©2007 Charles Taylor. Page updated: November 28, 2007.